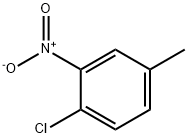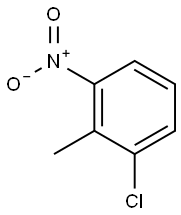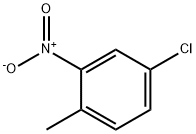
4-Chloro-2-nitrotoluene synthesis
- Product Name:4-Chloro-2-nitrotoluene
- CAS Number:89-59-8
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58
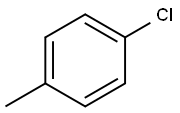
106-43-4
356 suppliers
$14.00/5g

89-59-8
212 suppliers
$23.00/25g
Yield:89-59-8 49.2%
Reaction Conditions:
with sulfuric acid;nitric acid in water at 50 - 55; for 2 h;
Steps:
9 4.5.9 4-Chloro-1-methyl-2-nitrobenzene (9)
H2O (3.3mL) was dispersed in 4-chlorotoluene (39.5mmol) and whilst stirring the mixture of 65% HNO3 (3.0mL) and 96% H2SO4 (13.2mL) was added drop wise at the temperature 50-55°C. Then, the reaction mixture was stirred at 55°C for 2h, H2O (50mL) was added and the mixture was extracted three times with CHCl3 (50mL). The organic phases were collected, dried over Na2SO4 and purified by column chromatography. Yield: 49.2%, yellow-white solid; mp: 37-38°C; IR: 1556 ( CC aromatic), 1521 (as NO2), 1481, 1451 ( CC aromatic), 1347 (s NO2) cm-1; 1H NMR (CDCl3, 300MHz): δ 7.96 (1H, d, J=2.2Hz, H3), 7.47 (1H, dd, J=8.2Hz, J=2.2Hz, H5), 7.29 (1H, d, J=8.2Hz, H6), 2.57 (3H, s, CH3); 13C NMR (CDCl3, 75MHz): δ 149.35, 133.81, 133.01, 132.39, 132.02, 124.67, 19.95.
References:
Kozic, Ján;Novák, Zdeněk;?ímal, Václav;Profant, Václav;Kune?, Ji?í;Vin?ová, Jarmila [Tetrahedron,2016,vol. 72,# 17,p. 2072 - 2083]
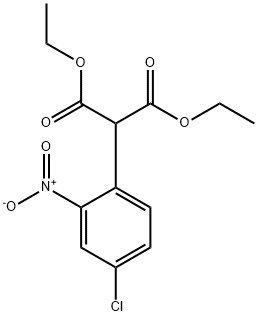
10565-16-9
14 suppliers
inquiry

89-59-8
212 suppliers
$23.00/25g
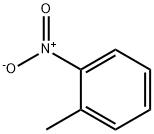
88-72-2
278 suppliers
$15.00/5g

89-59-8
212 suppliers
$23.00/25g