
5-(3-Nitrophenyl)-4H-1,2,4-triazol-3-amine synthesis
- Product Name:5-(3-Nitrophenyl)-4H-1,2,4-triazol-3-amine
- CAS Number:59301-20-1
- Molecular formula:C8H7N5O2
- Molecular Weight:205.17
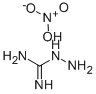
10308-82-4
161 suppliers
$39.79/5g
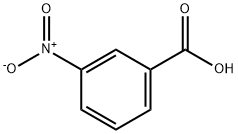
121-92-6
593 suppliers
$6.00/25g

59301-20-1
22 suppliers
$65.00/100mg
Yield:59301-20-1 81.4%
Reaction Conditions:
Stage #1: aminoguanidine nitratewith sodium hydroxide in methanol at 20; for 3 h;Inert atmosphere;
Stage #2: 3-nitrobenzoic acidwith N-ethyl-N,N-diisopropylamine;1,1'-carbonyldiimidazole in N,N-dimethyl-formamide at 0 - 20; for 3 h;Inert atmosphere;
Steps:
1 Step1: Synthesis of 3- (3-nitrophenyl) -1H-1, 2, 4-triazol-5-amine (Intermediate 1)
:[0123]A suspension of Aminoguanidinium nitrate (2.58g, 18.82mmol) and NaOH (789.79mg, 19.75mmol) in methanol (30 mL) was stirred at room temperature for 3 h, the solvent was evaporated off, and the residue was resolved by N, N-dimethylformamide (DMF) (30 mL) . A solution of 3-nitrobenzoic acid (3.0g, 17.95mmol) and N, N-Diisopropylethylamine (DIPEA) (2.55g, 19.73mmol) in N, N-dimethylformamide (DMF) (30 mL) was treated with N, N'-carbonyldiimidazole (CDI) (3.2g, 19.73mmol) at 0 . The solution was stirred at room temperature for 3 h. Then, it was added dropwise to the suspension. After the addition, the reaction mixture was stirred at room temperature for 3 h. The solvent was removed by rotatory evaporation, the residue was dissolved in water (100 mL) , and heated to reflux for 18 h. After cooling to room temperature, it was filtered, and the solid was re-crystallized from methanol to give 3- (3-nitrophenyl) -1H-1, 2, 4-triazol-5-amine (1) as yellow solid (3.0g, yield 81.5) . Mass spectrum (ESI) m/z calc. for C8H7N5O2[M+H]+206.06, found 206.20.1H NMR (400 MHz, d6-DMSO) δ 12.38 (s, 1H) , 8.65-8.60 (m, 1H) , 8.28 (d, J 7.8 Hz, 1H) , 8.22-8.16 (m, 1H) , 7.71 (t, J 8.0 Hz, 1H) , 6.22 (s, 2H) .
References:
WO2017/152842,2017,A1 Location in patent:Paragraph 0107; 0108
618-94-0
150 suppliers
$12.00/1mg

59301-20-1
22 suppliers
$65.00/100mg
121-90-4
24 suppliers
$24.00/25g

59301-20-1
22 suppliers
$65.00/100mg

90792-57-7
0 suppliers
inquiry

59301-20-1
22 suppliers
$65.00/100mg