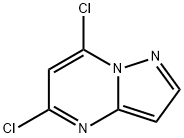
5,7-DICHLOROPYRAZOLO[1,5-A]PYRIMIDINE synthesis
- Product Name:5,7-DICHLOROPYRAZOLO[1,5-A]PYRIMIDINE
- CAS Number:57489-77-7
- Molecular formula:C6H3Cl2N3
- Molecular Weight:188.01
![Pyrazolo[1,5-a]pyrimidine-5,7(4H,6H)-dione](/CAS/GIF/57489-70-0.gif)
57489-70-0
![5,7-DICHLOROPYRAZOLO[1,5-A]PYRIMIDINE](/CAS/GIF/57489-77-7.gif)
57489-77-7
The general procedure for the synthesis of 5,7-dichloropyrazolo[1,5-a]pyrimidines from 7-hydroxy[1,5-a]pyrimidin-5(4H)-ones was as follows: trichlorophosphorus oxychloride (POCl3, 130 mL) was added to a reaction vial containing pyrazolo[1,5-a]pyrimidine-5,7-diol (10 g, 66 mmol). After the resulting suspension was cooled to 0°C, N,N-dimethylaniline (23 mL, 179 mmol) was slowly added. After the reaction mixture was warmed up to room temperature, the reaction was heated at 60°C for 16 h under the protection of nitrogen (N2). After completion of the reaction, the mixture was cooled to room temperature and concentrated to give a brown viscous liquid. This liquid was slowly poured into ice water and allowed to gradually warm to room temperature. Subsequently, the pH of the mixture was adjusted with saturated sodium bicarbonate (NaHCO3) solution to 8. The organic layer was extracted with dichloromethane (CH2Cl2, 4 x 50 mL), and the organic phases were combined and dried over anhydrous magnesium sulfate (MgSO4). The dried organic phase was concentrated under reduced pressure to give a brown liquid. Finally, the target product, 5,7-dichloropyrazolo[1,5-a]pyrimidine (10.7 g, 86% yield), was obtained as a white crystalline solid by fast column chromatography with the eluent ratio varied from 1:1 (v/v) dichloromethane:hexane gradient to 2:1 (v/v) dichloromethane:hexane. Mass spectrometry (MS) analysis showed m/z 189.36 [M + 1].
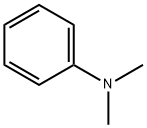
121-69-7
567 suppliers
$10.00/25mL
![Pyrazolo[1,5-a]pyrimidine-5,7(4H,6H)-dione](/CAS/GIF/57489-70-0.gif)
57489-70-0
59 suppliers
$21.00/100mg
![5,7-DICHLOROPYRAZOLO[1,5-A]PYRIMIDINE](/CAS/GIF/57489-77-7.gif)
57489-77-7
209 suppliers
$13.00/100mg
Yield:57489-77-7 80%
Reaction Conditions:
with trichlorophosphate
Steps:
5.A 5,7-Dichloropyrazolo[1,5-a]pyrimidine (J2)
5,7-Dichloropyrazolo[1,5-a]pyrimidine (J2)
POCl3 (10 mL, 109 mmol) was added to a mixture of J1 (800 mg, 5.29 mmol) and dimethyl aniline (1.8 g, 14.82 mmol) in an ice-cooled round-bottom flask.
The reaction mixture was stirred at room temperature for 10 min and then heated to 60 °C for 3 h.
The excess POCl3 was removed in vacuo, and the resulting residue was poured onto ice water.
The aqueous solution was basified to pH=9 with solid NaHCO3.
The mixture was extracted with EtOAc (3x100 mL).
The combined organics were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo.
The resulting residue was purified by gradient elution on silica gel (20 to 80% EtOAc in heptane) to afford the title compound as a white solid (810 mg, 81%).
1H NMR (300 MHz, CDCl3): δ 8.22 (d, J = 3.0 Hz, 1H), 7.00 (s, 1H), 6.76 (d, J = 3.0 Hz, 1H) ppm; LRMS m/z (M+H) 188.1 found, 188.0 required.
References:
EP2621926,2017,B1
![Pyrazolo[1,5-a]pyrimidine-5,7(4H,6H)-dione](/CAS/GIF/57489-70-0.gif)
57489-70-0
59 suppliers
$21.00/100mg
124-40-3
545 suppliers
$18.00/100ml
![5,7-DICHLOROPYRAZOLO[1,5-A]PYRIMIDINE](/CAS/GIF/57489-77-7.gif)
57489-77-7
209 suppliers
$13.00/100mg
1820-80-0
476 suppliers
$5.00/5g
105-53-3
791 suppliers
$5.00/25g
![5,7-DICHLOROPYRAZOLO[1,5-A]PYRIMIDINE](/CAS/GIF/57489-77-7.gif)
57489-77-7
209 suppliers
$13.00/100mg