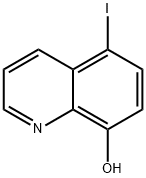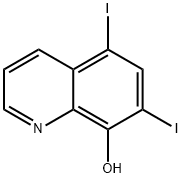
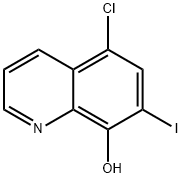
5,7-Diiodo-8-quinolinol synthesis
- Product Name:5,7-Diiodo-8-quinolinol
- CAS Number:83-73-8
- Molecular formula:C9H5I2NO
- Molecular Weight:396.95
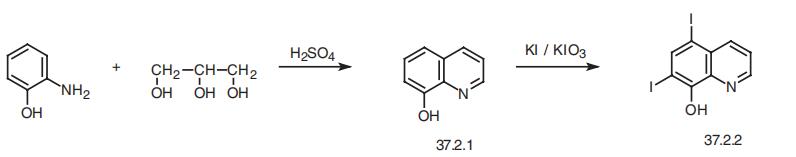
148-24-3
879 suppliers
$5.00/25g

83-73-8
230 suppliers
$6.00/250mg
Yield:83-73-8 87%
Reaction Conditions:
with 1-butyl-3-methyl-pyridinium dichloroiodate at 80; for 1 h;Inert atmosphere;
Steps:
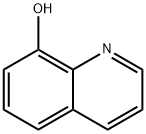
6.7 7) Synthesis of 5,7-diiodo-8-hydroxyquinoline:
8-Hydroxyquinoline (0.1 g, 0.69 mmol) and l-butyl-3-methylpyridinium dichloroiodate (BMPDCI) (0.31 g, 0.89 mmol) were added in 10 ml of one neck round bottomed flask in an inert atmosphere and then heated at 80 °C for lhr. After the reaction was completed (TLC), ethyl acetate (10ml) was added followed by addition of water (10ml). The entire reaction mixture was extracted with ethyl acetate (3x10ml). The combined organic layers were dried using sodium sulphate and concentrated on rotavapor to afford the crude product. This was further purified by silica gel column chromatography to afford 0.24 g (87 %) of pure 5,7- diiodo-8-hydroxyquinoline as a solid. (M.P. decomposes >210 °C) 1H NMR (DMSO-d6 δ/ppm): 8.88 (d, 1H, 7=4 Hz, Ar-H), 8.3 (s, 1H, Ar-H), 8.27-8.31 (dd, 1H, 7=1.3 Hz, 8.6 Hz, Ar-H), 7.75 (q, 1H, 7=4.2 Hz, 8.6 Hz, Ar-H).
References:
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH;SWAMY, Vincent Paul;DESHMUKH, Amarsinh Jayawant;GORE, Pranav Sopan;THULASIRAM, Hirekodathakallu V WO2016/113757, 2016, A1 Location in patent:Page/Page column 18-19

148-24-3
879 suppliers
$5.00/25g

83-73-8
230 suppliers
$6.00/250mg
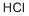
7647-01-0
0 suppliers
$10.00/10g

148-24-3
879 suppliers
$5.00/25g

7732-18-5
507 suppliers
$12.69/100ml
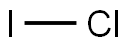
7790-99-0
259 suppliers
$48.72/25 G

83-73-8
230 suppliers
$6.00/250mg
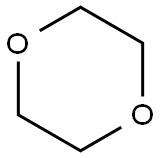
123-91-1
710 suppliers
$16.00/25g
130-26-7
321 suppliers
$19.00/5mg

7732-18-5
507 suppliers
$12.69/100ml
773-76-2
250 suppliers
$5.00/100mg

83-73-8
230 suppliers
$6.00/250mg