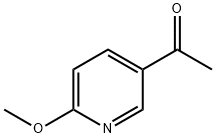
5-ACETYL-2-METHOXYPYRIDINE, 97% synthesis
- Product Name:5-ACETYL-2-METHOXYPYRIDINE, 97%
- CAS Number:213193-32-9
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
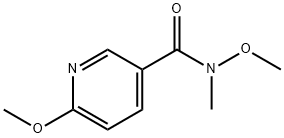
858600-08-5
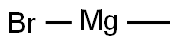
75-16-1

213193-32-9
Step 2: Amide (10.4 g, 53 mmol) was dissolved in tetrahydrofuran (THF, 100 mL) and the solution was cooled to 0°C. Methylmagnesium bromide (3M ether solution, 26.5 mL) was added slowly and dropwise at 0 °C. After the dropwise addition was completed, the reaction mixture was kept at 0°C and continued to be stirred for 1 hour. Subsequently, the reaction was quenched slowly with 1 M hydrochloric acid (aqueous solution). The mixture was extracted with ethyl acetate (EtOAc) and the organic layers were combined. The organic layer was washed with saturated brine and dried with anhydrous magnesium sulfate (MgSO4). After filtration to remove the drying agent, the filtrate was concentrated under reduced pressure to give 7.8 g of 5-acetyl-2-methoxypyridine in the form of a colorless solid in 97% yield.

67-56-1
790 suppliers
$7.29/5ml-f
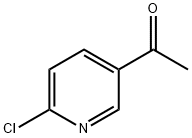
55676-22-7
270 suppliers
$7.00/100mg

213193-32-9
167 suppliers
$8.00/1g
Yield: 230 mg
Reaction Conditions:
with sodium methylate at 160; for 4 h;Microwave irradiation;
Steps:
32.1 1-(6-methoxypyrid-3-yl)ethanone
A mixture of 15 mL of methanol, 500 mg (2.89 mmol) of 1-(6-chloropyrid-3-yl)ethanone and 1.17 g (21.69 mmol) of sodium methoxide is heated in a microwave reactor at 160°C for 4 hours. The reaction medium is evaporated to dryness. After purification by chromatography on silica gel (eluent A B: heptane/EtOAc, gradient A/B: t 0 min 0% B, t 5 min 20% B, t 30 min 40% B), 230 mg of 1-(6-methoxypyrid-3-yl)ethanone were obtained, corresponding to the following characteristics: LC/MS (method G): ESI+ [M+H]+: m/z 152. tr (min) = 1.33. 1H NMR (300 MHz, δ in ppm, DMSO-d6): 2.50 (s, 3H), 3.94 (s, 3H), 6.92 (m, 1 H), 8.18 (m, 1 H), 8.83 (m, 1 H).
References:
SANOFI;EL-AHMAD, Youssef;FILOCHE-ROMME, Bruno;GANZHORM, Axel;MARCINIAK, Gilbert;MUZET, Nicolas;RONAN, Baptiste;VIVET, Bertrand;ZERR, Véronique WO2013/190123, 2013, A1 Location in patent:Page/Page column 106

858600-08-5
19 suppliers
inquiry
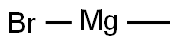
75-16-1
274 suppliers
$12.00/10ml

213193-32-9
167 suppliers
$8.00/1g
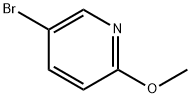
13472-85-0
523 suppliers
$8.00/1g
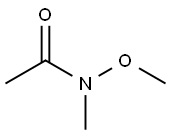
78191-00-1
287 suppliers
$6.00/5g

213193-32-9
167 suppliers
$8.00/1g

13472-85-0
523 suppliers
$8.00/1g

111-34-2
267 suppliers
$13.00/25mL

213193-32-9
167 suppliers
$8.00/1g

858600-08-5
19 suppliers
inquiry
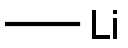
917-54-4
183 suppliers
$44.39/50ml

213193-32-9
167 suppliers
$8.00/1g