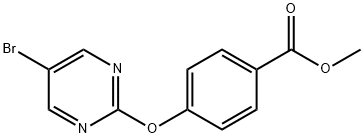
5-BROMO-2-[(4-METHOXYCARBONYL)PHENOXY]PYRIMIDINE synthesis
- Product Name:5-BROMO-2-[(4-METHOXYCARBONYL)PHENOXY]PYRIMIDINE
- CAS Number:926304-76-9
- Molecular formula:C12H9BrN2O3
- Molecular Weight:309.12
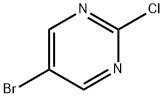
32779-36-5
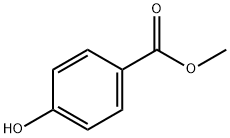
99-76-3
![5-BROMO-2-[(4-METHOXYCARBONYL)PHENOXY]PYRIMIDINE](/CAS/GIF/926304-76-9.gif)
926304-76-9
Methyl 4-hydroxybenzoate (260 mg, 1.71 mmol) was dissolved in N,N-dimethylformamide (DMF, 25 mL) at room temperature, followed by the addition of 60% sodium hydride (NaH, 75 mg, 1.9 mmol). The reaction mixture was stirred for 30 min. After that, 5-bromo-2-chloropyrimidine (300 mg, 1.55 mmol) was added and the reaction system was heated to 130 °C and the reaction was maintained for 0.5 hours. After standard post-treatment and purification steps, the target product methyl 4-(5-bromopyrimidin-2-yloxy)benzoate (428 mg, 89% yield) was obtained.

32779-36-5
634 suppliers
$5.00/5g

99-76-3
813 suppliers
$5.00/25g
![5-BROMO-2-[(4-METHOXYCARBONYL)PHENOXY]PYRIMIDINE](/CAS/GIF/926304-76-9.gif)
926304-76-9
48 suppliers
$54.00/1g
Yield:926304-76-9 89%
Reaction Conditions:
Stage #1: methyl 4-hydroxylbenzoatewith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: 5-Bromo-2-chloropyrimidine in N,N-dimethyl-formamide at 130; for 0.5 h;
Steps:
To a solution of methyl 4-hydroxybenzoate (260 mg, 1.71 mmol) in DMF (25 mL) at rt was added NaH (60%, 75 mg, 1.9 mmol) and the mixture was stirred for 30 minutes. 5- Bromo-2-chloropyrimidine (300 mg, 1.55 mmol) was added and the mixture heated to 130 0C for 0.5 hours. Standard work-up and purification afforded 4-(5-bromo~pyrimidin-2-yloxy)- benzoic acid methyl ester (428 mg, 89%).
References:
WO2007/22371,2007,A2 Location in patent:Page/Page column 65