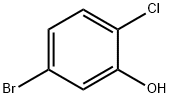
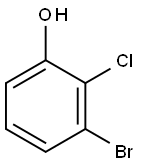
5-Bromo-2-chlorophenol synthesis
- Product Name:5-Bromo-2-chlorophenol
- CAS Number:183802-98-4
- Molecular formula:C6H4BrClO
- Molecular Weight:207.45
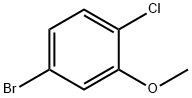
16817-43-9

183802-98-4
General procedure for the synthesis of 5-bromo-2-chlorophenol from 5-bromo-2-chloroanisole: BBr3 (8.2 mL, 84.9 mmol) was slowly added dropwise to a solution of 5-bromo-2-chloroanisole (18.26 g, 82.4 mmol) in dichloromethane (45 mL) under stirring conditions at 0-5 °C. The reaction mixture was stirred continuously for 4 hours at room temperature. Upon completion of the reaction, the mixture was carefully poured into a pre-cooled 2N NaOH/ice mixture and washed twice with tert-butyl methyl ether (TBME). The aqueous phase was acidified with 2N HCl to a suitable pH and then extracted again with TBME twice. All organic phases were combined and dried with anhydrous Na2SO4, followed by evaporation of the solvent under reduced pressure to afford the target product 5-bromo-2-chlorophenol as colorless crystals (16.35 g, 95% yield). The product was confirmed by 1H-NMR (400 MHz, DMSO-d6) and mass spectrometry (ES+) analysis: 1H-NMR δ (ppm): 6.97 (dd, 1H), 7.09 (d, 1H), 7.26 (d, 1H), 10.65 (bs, 1H, OH); MS (m/z) ES+: 210 (15), 208 (70, M+) , 206 (50), 179 (20), 177 (15), 63 (100).

16817-43-9
221 suppliers
$5.00/1g

183802-98-4
176 suppliers
$6.00/1g
Yield:183802-98-4 98%
Reaction Conditions:
Stage #1:5-Bromo-2-chloroanisole with boron tribromide in dichloromethane at 0 - 20; for 20.6667 h;
Stage #2: with water in dichloromethane at 0; for 0.5 h;
Steps:
5
Preparation 5: 5 -Bromo-2-chloro-phenol; A solution of 5 -bromo-2-chloroanisole (20 g, 90.3 mmol) in dichloromethane (100 ml) at 0 "C under nitrogen was treated with borontribromide (1M in dichloromethane, 100 mL, 0.1 mol) dropwise over 2.5 hours. After 10 minutes the reaction was allowed to warm to RT and stirred for 18 hours. The reaction mixture was poured into water (200 mL) and ice (200 mL) and stirred for 30 minutes, dichloromethane (100 mL) was added and the organics separated. The aqueous phase was saturated with sodium chloride and re -extracted with dichloromethane (2x 200 mL). The combined organics were dried (MgSO 4) to furnish a white solid (18.34 g, 98 %).
References:
PFIZER LIMITED WO2007/91152, 2007, A1 Location in patent:Page/Page column 43
591-20-8
349 suppliers
$10.00/1g
13659-24-0
146 suppliers
$11.00/1g

183802-98-4
176 suppliers
$6.00/1g
863870-87-5
117 suppliers
$7.00/100mg

16817-43-9
221 suppliers
$5.00/1g

183802-98-4
176 suppliers
$6.00/1g

108-95-2
786 suppliers
$14.00/25g

591-20-8
349 suppliers
$10.00/1g

13659-24-0
146 suppliers
$11.00/1g

183802-98-4
176 suppliers
$6.00/1g
18282-59-2
330 suppliers
$6.00/5g

124-41-4
730 suppliers
$12.00/25g

183802-98-4
176 suppliers
$6.00/1g