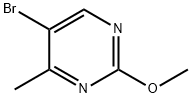
5-BroMo-2-Methoxy-4-MethylpyriMidine synthesis
- Product Name:5-BroMo-2-Methoxy-4-MethylpyriMidine
- CAS Number:38696-23-0
- Molecular formula:C6H7BrN2O
- Molecular Weight:203.04

67-56-1
790 suppliers
$7.29/5ml-f
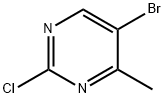
633328-95-7
102 suppliers
$12.00/250mg

124-41-4
730 suppliers
$12.00/25g

38696-23-0
44 suppliers
$46.00/100mg
Yield: 500 mg
Reaction Conditions:
at 25; for 2 h;
Steps:
A fourth exemplary Intermediate C, Intermediate C-4, may be used to synthesize compounds of formula I or formula II, wherein R1 is disubstitued heteroaryl. A mixture of sodium (111 mg, 4.82 mmol, 1.00 equiv) in methanol (772 mg, 24.1 mmol, 975. μL, 5.00 equiv) was stirred at 25 °C for 0.5 h. To this solution was added 5-bromo-2-chloro-4-methyl- pyrimidine (1.00 g, 4.82 mmol, 1.00 equiv) and the mixture was stirred at 25 °C for 2 h. The reaction was quenched upon the addition of water (5 mL). The aqueous phase was extracted with ethyl acetate (10.0 mL × 3) and the combined organic phase was washed with brine (10.0 mL × 3), dried over anhydrous Na2SO4, filtered and concentrated in vacuo to afford 5-bromo- 2-methoxy-4-methyl-pyrimidine (500 mg, 2.46 mmol, 51.1% yield) as a red oil. LC-MS: [M+1] 203.1.
References:
MIRATI THERAPEUTICS, INC.;KETCHAM, John, Michael;BURNS, Aaron, Craig;MARX, Matthew, Arnold WO2020/219448, 2020, A1 Location in patent:Paragraph 0347

67-56-1
790 suppliers
$7.29/5ml-f

633328-95-7
102 suppliers
$12.00/250mg

38696-23-0
44 suppliers
$46.00/100mg

633328-95-7
102 suppliers
$12.00/250mg

124-41-4
730 suppliers
$12.00/25g

38696-23-0
44 suppliers
$46.00/100mg