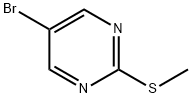
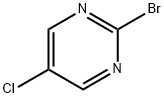
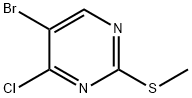
5-BROMO-2-(METHYLTHIO)PYRIMIDINE synthesis
- Product Name:5-BROMO-2-(METHYLTHIO)PYRIMIDINE
- CAS Number:14001-67-3
- Molecular formula:C5H5BrN2S
- Molecular Weight:205.08
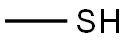
74-93-1
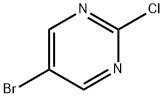
32779-36-5

14001-67-3
Step 1: Synthesis of 5-bromo-2-methylmercaptopyrimidine To a stirred solution of 5-bromo-2-chloropyrimidine (0.3 g, 1.563 mmol) in dimethylformamide (DMF, 10 mL) was slowly added methyl mercaptan (0.1 mL, 1.563 mmol) at room temperature. The reaction mixture was then heated to 50 °C with continuous stirring for 3 hours. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was quenched by addition of water and extracted with ethyl acetate (EtOAc). The organic phases were combined and dried over anhydrous sodium sulfate (Na2SO4), followed by evaporation of the solvent under reduced pressure to give the crude product. The crude product was purified by silica gel (100-200 mesh) column chromatography using 5% ethyl acetate/hexane as eluent, resulting in purified 5-bromo-2-methylmercaptopyrimidine (0.240 g, 75% yield) as a white solid. Mass spectrometry (MS) analysis showed that [M++1] was 205.1.
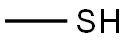
74-93-1
143 suppliers
$454.02/5MG

32779-36-5
635 suppliers
$5.00/5g

14001-67-3
144 suppliers
$8.00/250mg
Yield:14001-67-3 75%
Reaction Conditions:
in N,N-dimethyl-formamide at 50; for 3 h;
Steps:
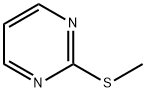
24.1 Step 1: Synthesis of 5-bromo-2-(methylthio)pyrimidine
Step 1:
Synthesis of 5-bromo-2-(methylthio)pyrimidine
To a stirred solution of 5-bromo-2-chloropyrimidine (0.3 g, 1.563 mmol) in DMF (10 mL) was added methyl mercaptan (0.1 mL, 1.563 mmol) at room temperature and heat at 50° C. for 3 h.
Completion of reaction was monitored by TLC.
Reaction mixture was quenched by addition of water, extracted with EtOAc.
Organic portions were combined, dried over Na2SO4, evaporated under reduced pressure to obtain crude which was purified by column chromatography using silica gel (100-200 mesh); eluent 5% ethyl acetate/hexane to obtain pure product 5-bromo-2-(methylthio)pyrimidine (0.240 g, 75%) as white solid.
MS: 205.1[M++1]
References:
US2017/291910,2017,A1 Location in patent:Paragraph 0496-0498

32779-36-5
635 suppliers
$5.00/5g
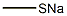
5188-07-8
298 suppliers
$13.00/5g

14001-67-3
144 suppliers
$8.00/250mg
124405-67-0
111 suppliers
$20.00/100mg
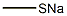
5188-07-8
298 suppliers
$13.00/5g

14001-67-3
144 suppliers
$8.00/250mg
823-09-6
65 suppliers
$56.00/250mg

14001-67-3
144 suppliers
$8.00/250mg
63810-78-6
172 suppliers
$9.00/250mg

14001-67-3
144 suppliers
$8.00/250mg