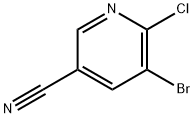
5-bromo-6-chloronicotinonitrile synthesis
- Product Name:5-bromo-6-chloronicotinonitrile
- CAS Number:71702-01-7
- Molecular formula:C6H2BrClN2
- Molecular Weight:217.45
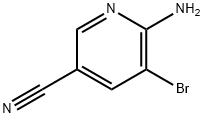
477871-32-2

71702-01-7
Step 2; 5-Bromo-6-chloronicotinonitrile; To a solution of anhydrous CuCl2 (77 mg, 0.58 mmol) in acetonitrile (3 mL) was added tert-butyl cyanide (0.72 mmol). The reaction mixture was heated to 65 °C, followed by the addition of a suspension of acetonitrile (2 mL) of the above intermediate 6-amino-5-bromonicotinonitrile (96 mg, 0.48 mmol). The reaction mixture was stirred continuously for 3 hours. After completion of the reaction, the mixture was cooled to room temperature, poured into 3M HCl solution and extracted with ethyl acetate (EtOAc). The organic layer was dried with anhydrous Na2SO4 and subsequently concentrated under reduced pressure to remove the solvent. The product was purified by silica gel fast column chromatography (eluent: hexane/ethyl acetate, 2:1, v/v) to afford 5-bromo-6-chloronicotinonitrile (55 mg, 55% yield).1H-NMR (DMSO-d6, 300 MHz): δ 8.93 (s, 1H), 8.90 (s, 1H).

477871-32-2
94 suppliers
inquiry

71702-01-7
91 suppliers
inquiry
Yield:71702-01-7 55%
Reaction Conditions:
with tert.-butylnitrite;copper dichloride in acetonitrile at 65; for 3 h;
Steps:
36.2
Step 2; 5-Bromo-6-chloro-nicotinonitrile; To a solution of anhydrous CuCl2 (77 mg, 0.58 mmol) in CH3CN (3 mL) add tert- BuONO (0.72 mmol). Heat the mixture at 65°C and then add a suspension of the intermediate above (96 mg, 0.48 mmol) in CH3CN (2 mL). Stir the mixture for 3 hours. Cool at room temperature. Pour into HCI 3M and extract with EtOAc. Dry the organic layer over Na2SO4. Eliminate the solvent. Purify by flash chromatography on silica gel (eluent: hexane/EtOAc 2/1) to afford the title compound (55 mg, 55%). 'H-NMR (DMSO, 300 MHz): 8.93 (s, 1H), 8.90 (s, 1H).
References:
WO2005/90337,2005,A1 Location in patent:Page/Page column 43