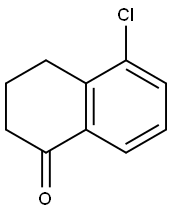
5-Chloro-1-tetralone synthesis
- Product Name:5-Chloro-1-tetralone
- CAS Number:26673-30-3
- Molecular formula:C10H9ClO
- Molecular Weight:180.63
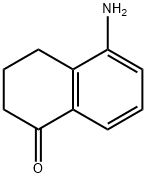
41823-28-3

26673-30-3
The general procedure for the synthesis of 5-chloro-3,4-dihydronaphthalen-1(2H)-one from 5-amino-3,4-dihydro-2H-1-naphthalenone was as follows: 5-amino-3,4-dihydronaphthalen-1(2H)-one (1 g, 6.2 mmol) was dissolved in a mixture of HCl (36%, 2 mL) and EtOH (8 mL) at 0 °C, and NaNO2 was slowly added aqueous solution (0.582 g, 8.4 mmol dissolved in 1 mL of water) and stirred for 15 min. Subsequently, the reaction mixture was added dropwise to a solution of CuCl (0.3 g, 3.1 mmol) in HCl (36%, 10 mL) that was preheated to 95 °C and kept at that temperature for 15 minutes. Upon completion of the reaction, the solution was cooled to room temperature, diluted with water and extracted with EtOAc. The organic phases were combined, washed with saturated NaHCO3 solution, dried over MgSO4, filtered and purified by silica gel column chromatography with 40-50% EtOAc/hexane as eluent. The target product 5-chloro-3,4-dihydronaphthalen-1(2H)-one (0.7 g, 62% yield) was finally obtained. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.53 (dd, J = 7.8, 1.2 Hz, 1H), 7.14 (tt, J = 7.8, 0.7 Hz, 1H), 6.88 (dd, J = 7.8, 1.2 Hz, 1H), 3.70 (s, 2H), 2.69 (t, J = 6.2 Hz, 2H), and 2.66-2.60 (m, 2H), 2.30-2.07 (m, 2H).

41823-28-3
26 suppliers
inquiry

26673-30-3
70 suppliers
$55.00/100mg
Yield: 62%
Reaction Conditions:
Stage #1:5-amino-3,4-dihydronaphthalen-1(2H)-one with hydrogenchloride;sodium nitrite in ethanol;water at 0; for 0.25 h;
Stage #2: with copper(l) chloride in ethanol;water at 95; for 0.25 h;
Steps:
6.3 6.3. Synthesis of 5-chloro-3,4-dihydronaphthalen-l(2H)-one
5-amino-3,4-dihydronaphthalen-l(2H)-one (1 g, 6.2 mmol) in HCl (36%, 2 mL) and EtOH (8 mL) at 0 °C was treated with an aqueous solution of NaN02 (0.582 g, 8.4 mmol in 1 mL water for 15 min. This mixture was added to a solution of CuCl (0.3 g, 3.1 mmol) in HCl (36%, 10 mL) at 95 °C and kept at this temperature for 15 min. The solution was cooled down to room temperature, diluted with water, and extracted with EtOAc. The organic phase was collected, washed with sat. NaHCC , dried over MgSC , and purified by column chromatography with 40-50% EtOAc:Hex on silica gel. Desired compound was afforded. (0.7 g, 62%). NMR (400 MHz, chloroform-d) δ 7.53 (dd, / = 7.8, 1.2 Hz, 1H), 7.14 (tt, / = 7.8, 0.7 Hz, 1H), 6.88 (dd, / = 7.8, 1.2 Hz, 1H), 3.70 (s, 2H), 2.69 (t, / = 6.2 Hz, 2H), 2.66 - 2.60 (m, 2H), 2.30 - 2.07 (m, 2H).
References:
THE BOARD OF REGENTS OF THE UNIVERSITY OF TEXAS SYSTEM;GAO, Jinming;LUI, Yuliang;WEI, Qi;MA, Xinpeng;HUANG, Gang WO2017/106624, 2017, A1 Location in patent:Paragraph 00141
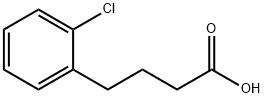
68449-31-0
23 suppliers
inquiry

26673-30-3
70 suppliers
$55.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

26673-30-3
70 suppliers
$55.00/100mg
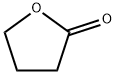
96-48-0
0 suppliers
$21.10/25g
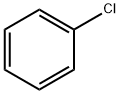
108-90-7
685 suppliers
$10.00/25G

26673-30-3
70 suppliers
$55.00/100mg
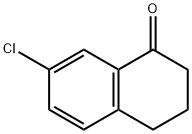
26673-32-5
158 suppliers
$7.00/100mg
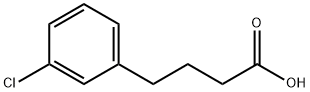
22991-05-5
28 suppliers
inquiry

26673-30-3
70 suppliers
$55.00/100mg