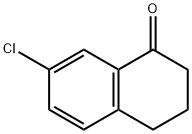
7-Chloro-1-tetralone synthesis
- Product Name:7-Chloro-1-tetralone
- CAS Number:26673-32-5
- Molecular formula:C10H9ClO
- Molecular Weight:180.63
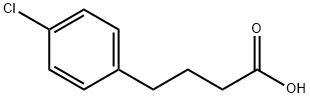
4619-18-5

26673-32-5
General procedure for the synthesis of 7-chloro-3,4-dihydro-2H-1-naphthalenone from 4-(4-chlorophenyl)butyric acid: polyphosphoric acid (20 g, excess) was placed in a beaker and heated to 90 °C. 4-(4-Chlorophenyl)butyric acid (3 g, 17 mmol) was added in batches. The reaction mixture was stirred for 5 minutes and then polyphosphoric acid (20 g, excess) was added and continued to be heated at 90°C for 5 minutes. The resulting thick orange viscous oil was cooled to 60 °C, followed by the slow addition of water. After completion of the reaction, the mixture was cooled to room temperature and extracted with ethyl acetate (EtOAc). The organic phase was washed sequentially with water, 1N NaOH solution and water, dried with anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to give a white solid product (2.5 g, 81% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 2.12-2.18 (m, 2H), 2.67 (t, J = 6.8 Hz, 2H), 2.95 (t, J = 6.8 Hz, 2H), 7.22 (d, J = 8.0 Hz, 1H), 7.42 (dd, J = 10.2, 2.4 Hz, 1H), 7.85 (d , J = 2.4 Hz, 1H).
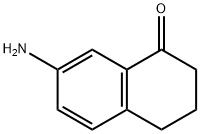
22009-40-1
119 suppliers
$76.00/1g

26673-32-5
159 suppliers
$7.00/100mg
Yield:26673-32-5 550 mg (3.05 mmol, 98%)
Reaction Conditions:
with sodium nitrite;copper(l) chloride in hydrogenchloride;water;
Steps:
35.B Step B:
Step B: 7-chloro-1-tetralone 7-Amino-1-tetralone (500 mg, 3.1 mmol) was suspended in 3 mL of water and treated with 3 mL of concentrated hydrochloric acid with stirring. The mixture was cooled in an ice bath and treated dropwise with vigorous stirring with a solution of 241 mg of sodium nitrite in 1.5 mL of water (3.5 mmol, 1.1 eq). The mixture was stirred at 0°-5° for 15 minutes then added dropwise to a cold solution of 366 mg of CuCl (3.7 mmol, 1.2 eq) in 6 mL of concentrated hydrochloric acid. The mixture was stirred for 5 minutes at 0° and 1 hour at room temperature. The mixture was extracted with methylene chloride (3*15 mL); the combined extracts were washed with brine, dried over magnesium sulfate, filtered and evaporated to dryness under vacuum at room temperature to give 550 mg (3.05 mmol, 98%) of the product. 1 H NMR (300 MHz, CDCl3): 2.16 (m,2H), 2.67 (t,6 Hz,2H), 2.95 (t,6 Hz,2H), 7.22 (d,8 Hz,1H), 7.44 (dd;2,8 Hz;1H), 8.01 (d,2 Hz,1H). FAB-MS: calculated for C10 H9 ClO 180; found 181 (M+H,10%).
References:
US5206235,1993,A

4619-18-5
74 suppliers
$17.00/250mg

26673-32-5
159 suppliers
$7.00/100mg

4619-18-5
74 suppliers
$17.00/250mg

26673-32-5
159 suppliers
$7.00/100mg
529-34-0
480 suppliers
$6.00/10g
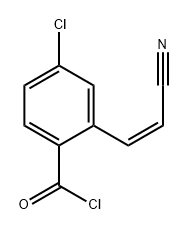
56820-67-8
0 suppliers
inquiry

26673-32-5
159 suppliers
$7.00/100mg
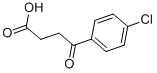
3984-34-7
146 suppliers
$7.00/1g

26673-32-5
159 suppliers
$7.00/100mg