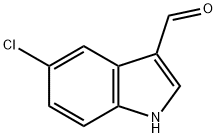
5-Chloroindole-3-carboxaldehyde synthesis
- Product Name:5-Chloroindole-3-carboxaldehyde
- CAS Number:827-01-0
- Molecular formula:C9H6ClNO
- Molecular Weight:179.6
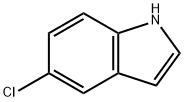
17422-32-1

827-01-0
The general procedure for the synthesis of 5-chloroindole-3-carbaldehyde from 5-chloroindole was as follows: phosphorus trichloride (4.6 mL, 49 mmol) was added slowly and dropwise to stirred dimethylformamide (20 mL) at 0 °C. After the dropwise addition, the reaction mixture was continued to be stirred for 10 minutes. Subsequently, a solution of 5-chloroindole (5.0 g, 33 mmol) dissolved in dimethylformamide (5 mL) was added slowly dropwise. After completion of the dropwise addition, the reaction mixture was heated to 40°C and kept at this temperature for 45 minutes. After completion of the reaction, the mixture was cooled to room temperature and then treated with a solution of sodium hydroxide (5.9 g, 148 mmol) in water (20 mL). Next, the mixture was heated to 50°C, held for 10 minutes and then cooled to room temperature. The reaction mixture was poured into crushed ice (100 mL) and the precipitate was collected by filtration. Finally, the filter cake was recrystallized by methanol to give the white solid product 5-chloroindole-3-carboxaldehyde (3.5 g, 59% yield) with a melting point of 215-216 °C. Elemental analysis measured values: C, 60.13; H, 3.40; N, 7.75%. Consistent with theoretical values (C9H6ClNO): C, 60.19; H, 3.37; N, 7.79%.
100-97-0
0 suppliers
$14.00/250G

17422-32-1
382 suppliers
$6.00/1g

827-01-0
155 suppliers
$9.00/250mg
Yield:827-01-0 83%
Reaction Conditions:
with water;iodine in N,N-dimethyl-formamide at 150; under 5171.62 Torr; for 0.133333 h;Flow reactor;
Steps:
3. C3-formylation of indoles in the continuous flow reactor
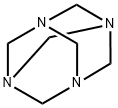
General procedure: The continuous flow reactor system consisted of the dual pump (KP-22-13, SS), PTFE tubing (length: 16 m, ID: 1 mm), T-junction (1.25 mm thru-hole), back pressure regulator (40, 75, 100, 250 psi), oil bath and hot plate. In this experiment, we used BOLA PTFE tubing to experiment at high temperature. In order to react stably at the temperature above the boiling point of water, looked for the maximum temperature conditions according to the pressure of the back pressure regulator in which the bubble does not occur. The reaction started in the concentration of indole (1.0 mmol), HMTA (2.0 mmol) and I 2 (1.0 mmol) in solvent (10 mL). Since this reaction proceeds in a single phase of liquid, the reaction time was set by the inner volume of tubing and the flow rate in the continuous flow reactor. After the reaction is over, the product was quantified using 1 H NMR and the NMR yield was obtained.
References:
Sung, Ha Kyoung;Kim, Dong Hyun;Kim, Joon Seok;Park, Chan Pil [Bulletin of the Korean Chemical Society,2021,vol. 42,# 3,p. 388 - 392] Location in patent:supporting information

50-00-0
896 suppliers
$10.00/25g

17422-32-1
382 suppliers
$6.00/1g

827-01-0
155 suppliers
$9.00/250mg

17422-32-1
382 suppliers
$6.00/1g

827-01-0
155 suppliers
$9.00/250mg

17422-32-1
382 suppliers
$6.00/1g
298-12-4
476 suppliers
$5.00/25g

827-01-0
155 suppliers
$9.00/250mg
877-03-2
231 suppliers
$6.00/1g

827-01-0
155 suppliers
$9.00/250mg