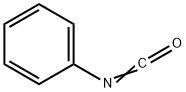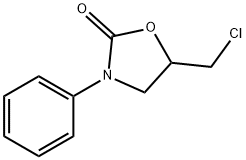
5-(chloromethyl)-3-phenyl-2-Oxazolidinone synthesis
- Product Name:5-(chloromethyl)-3-phenyl-2-Oxazolidinone
- CAS Number:711-85-3
- Molecular formula:C10H10ClNO2
- Molecular Weight:211.64

169268-95-5
0 suppliers
inquiry

711-85-3
13 suppliers
$65.00/25mg
Yield:711-85-3 86%
Reaction Conditions:
with N-chloro-succinimide;2,2'-azobis(isobutyronitrile) in acetonitrile; for 48 h;Inert atmosphere;Reflux;chemoselective reaction;Reagent/catalyst;Solvent;Time;
Steps:
4.1. General synthetic procedure of 2aX and 2bXs
Compound 1 (100 mg, 0.429 mmol) was dissolved in organic solvent (10 mL) and then the halogenating agent was added into the solution under nitrogen atmosphere. In case of Cl2, the mixture was neutralised with saturated NaHCO3. In case of NCS and NBS, cold water was added to the mixture until the solution turned turbid. In the case of Br2, I2, NIS, and KI/KIO3, saturated aqueous solution Na2S2O3 was added to the mixture until the orange or darkbrown solution turned to a colorless solution which was then neutralised with saturated NaHCO3. In the case of ICl, the mixture was added to 40% NaHSO3 until a dark brown solution turned to a colorless solution. After that, the mixture in each case was extracted with EtOAc (3 x 10 mL). The combined organic layers were washed with brine, dried over anhydrous MgSO4, filtered and evaporated in vacuo to yield the crude product as a pale yellow oil. Purification was accomplished by column chromatography (SiO2) eluting with hexane/EtOAc (85:15) to produce 2aX or/and 2bX as a white solid. 5-(Chloromethyl)-3-phenyloxazolidin-2-one (2aCl): Obtained as a yellow solid, m.p. 72-74 °C; Rf (40% EtOAc/Hexane) 0.33; νmax (liquid film) 3600-3100 (br), 3029, 2957, 1731, 1594, 1501, 1479,1408 cm1; δH (400 MHz, CDCl3) 7.55 (2H, d, J 7.8 Hz, Ar-Hortho), 7.37(2H, t, J 7.6 Hz, Ar-Hmeta), 7.16 (1H, t, J 7.4, Ar-Hpara), 4.90-4.84 (1H,m, NCH2CHCH2Cl), 4.18 (1H, t, J 9.0 Hz, NCHAHBCHCH2Cl), 3.97 (1H,dd, J 5.5, 9.0 Hz, NCHAHBCHCH2Cl), 3.81e3.72 (2H, m,NCH2CHCH2Cl); δC (100 MHz, CDCl3) 153.9, 137.7,129.2,124.3, 118.3,70.8, 48.1, 44.5; HRMS: MNa, found 234.0354. C10H10ClNNaO2 requires 234.0292.
References:
Paisuwan, Waroton;Chantra, Thanakrit;Rashatasakhon, Paitoon;Sukwattanasinitt, Mongkol;Ajavakom, Anawat [Tetrahedron,2017,vol. 73,# 24,p. 3363 - 3367]
2651-82-3
3 suppliers
inquiry

711-85-3
13 suppliers
$65.00/25mg
103-72-0
247 suppliers
$10.00/1g

106-89-8
778 suppliers
$9.00/10g

711-85-3
13 suppliers
$65.00/25mg
![Benzenamine, N-[5-(chloromethyl)-1,3-oxathiolan-2-ylidene]-](/CAS/20210305/GIF/1081977-30-1.gif)
1081977-30-1
0 suppliers
inquiry

62-53-3
688 suppliers
$10.00/1g

711-85-3
13 suppliers
$65.00/25mg