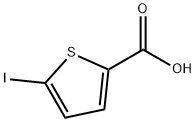
5-Iodo-thiophene-2-carboxylic acid synthesis
- Product Name:5-Iodo-thiophene-2-carboxylic acid
- CAS Number:60166-85-0
- Molecular formula:C5H3IO2S
- Molecular Weight:254.05
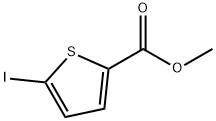
88105-22-0

60166-85-0
General procedure for the synthesis of 5-iodothiophene-2-carboxylic acid (P-2) from methyl 5-iodothiophene-2-carboxylate (P-1): to a 17.5 mL aqueous solution of lithium hydroxide (0.45 g, 11.19 mmol) was added a 35 mL tetrahydrofuran solution of compound P-1 (1.0 g, 3.73 mmol). The reaction mixture was stirred at room temperature for 4 h. The pH was subsequently adjusted to 4 with acid and extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 0.86 g (91% yield) of the target compound P-2 as a white solid.1H NMR (400 MHz, CD3OD) δ 7.35 (d, J = 3.8 Hz, 1H), 7.43 (d, J = 3.9 Hz, 1H).

88105-22-0
33 suppliers
$13.00/250mg

60166-85-0
48 suppliers
$76.00/100mg
Yield:60166-85-0 91%
Reaction Conditions:
with lithium hydroxide in tetrahydrofuran;water;
Steps:
1 5-Iodo-thiophene-2-carboxylic acid (P-2).
5-Iodo-thiophene-2-carboxylic acid (P-2). To a solution of lithium hydroxide (0.45 g, 11.19 mmol) in 17.5 mL of water was added compound P-1 (1.0 g, 3.73 mmol) in 35 mL of tetrahydrofuran. The resulting mixture was stirred for 4 hours at room temperature and was then acidified to pH 4 and extracted with ethyl acetate. The organic layer was dried (MgSO4), filtered and evaporated to yield 0.86 g (91% yield) of compound P-2 as a white solid. 1H NMR (400 MHz, CD3OD) d 7.35 (d, J=3.8 Hz, 1H), 7.43 (d, J=3.9 Hz, 1H).
References:
US6274620,2001,B1
527-72-0
503 suppliers
$5.00/10g

64-17-5
825 suppliers
$10.00/50g
2314-97-8
186 suppliers
$40.00/25g

60166-85-0
48 suppliers
$76.00/100mg
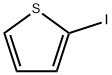
3437-95-4
251 suppliers
$6.00/5g

60166-85-0
48 suppliers
$76.00/100mg
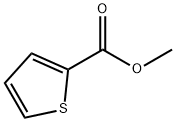
5380-42-7
177 suppliers
$6.00/1g

60166-85-0
48 suppliers
$76.00/100mg
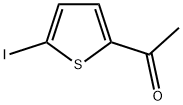
30955-94-3
83 suppliers
$15.00/250mg

60166-85-0
48 suppliers
$76.00/100mg