5-METHOXY-2,3-DIMETHYL-1H-INDOLE synthesis
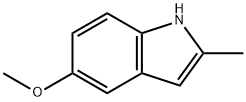
- Product Name:5-METHOXY-2,3-DIMETHYL-1H-INDOLE
- CAS Number:828-94-4
- Molecular formula:C11H13NO
- Molecular Weight:175.23
Yield:828-94-4 94%
Reaction Conditions:
with acetic acid for 1 h;Reflux;Concentration;
Steps:
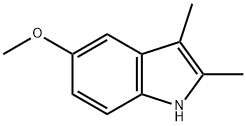
1.20 Preparation of 5-methoxy-2, 3-dimethyl-] H-indole
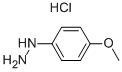
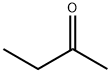
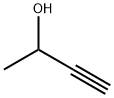
The mixture of (4-methoxyphenyl)hydrazine hydrochloride (10.5 g, 60 mmol) and butan-2-one (10.7 mL, 120 mmol) in acetic acid (200 mL) was heated to reflux for one hour. When cooled, the acetic acid was removed and the residue was dissolved in ethyl acetate (200 mL), washed with saturated NaHCO3 and brine, dried over Na2504 and concentrated in vacuum to give 5-methoxy-2,3-dimethyl- 1H-indole as a yellow solid (9.80 g, 94%).‘H NMR (400 MHz, CDC13): 7.58 (br s, 1H), 7.14 (d, J = 8.8 Hz, 1H), 6.92 (d, J= 1.2 Hz, 1H), 6.76 (dd, J= 8.4 Hz, 2.4 Hz, 1H), 3.86 (s, 3H), 2.35 (s, 3H), 2.19 (s, 3H). LCMS: m/z 176.1 [M+Hjt
References:
WO2016/187667,2016,A1 Location in patent:Page/Page column 122