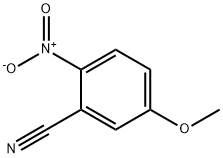
5-METHOXY-2-NITROBENZONITRILE synthesis
- Product Name:5-METHOXY-2-NITROBENZONITRILE
- CAS Number:38469-84-0
- Molecular formula:C8H6N2O3
- Molecular Weight:178.14

41994-92-7

38469-84-0
Step 2: Synthesis of 5-methoxy-2-nitrobenzonitrile To a 100 mL round bottom flask was added 5-methoxy-2-nitrobenzamide (2.1 g, 0.01 mol), trifluoroacetic anhydride (2.2 mL), triethylamine (2.9 mL) and dichloromethane (30 mL). The reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the reaction mixture was washed with distilled water (30 mL × 2). The organic layer was separated and dried over anhydrous sodium sulfate and subsequently concentrated under reduced pressure to give 1.75 g (92% yield) of white solid product. Mass spectrometry (MS) showed m/z: 179 ([M + H]+).
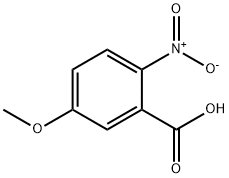
1882-69-5
290 suppliers
$6.00/5g

38469-84-0
74 suppliers
inquiry
Yield:38469-84-0 92%
Reaction Conditions:
with triethylamine;trifluoroacetic anhydride in dichloromethane at 20; for 1 h;
Steps:
101.2
A 100 mL round bottom flask was charged with 5-methoxy-2- nitrobenzamide (2.1 g, 0.01 mol), trifluoroacetic anhydride (2.2 mL), triethylamine (2.9 mL) and CH2C12 (30 mL). The resulting solution was stirred at room temperature for 1 h. Work-up: the reaction solution was washed with H20 (30 mL x 2). The organic layer was dried over anhydrous Na2S04 and concentrated in vacuo, to give 1.75 g (92%) of the product as white solid. MS m/z: 179 (M+H+).
References:
WO2011/112731,2011,A2 Location in patent:Page/Page column 143

41994-92-7
11 suppliers
$45.00/100mg

38469-84-0
74 suppliers
inquiry

67-56-1
791 suppliers
$9.00/25ml
50594-78-0
110 suppliers
$6.00/250mg

38469-84-0
74 suppliers
inquiry
20357-24-8
119 suppliers
$8.00/250mg

38469-84-0
74 suppliers
inquiry
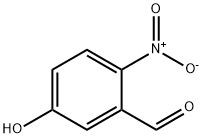
42454-06-8
217 suppliers
$8.00/1g

38469-84-0
74 suppliers
inquiry