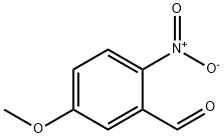
5-Methoxy-2-nitrobenzaldehyde synthesis
- Product Name:5-Methoxy-2-nitrobenzaldehyde
- CAS Number:20357-24-8
- Molecular formula:C8H7NO4
- Molecular Weight:181.15
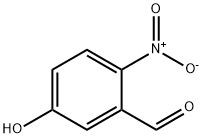
42454-06-8

74-88-4

20357-24-8
Step 1. Synthesis of 5-methoxy-2-nitrobenzaldehyde: 5-hydroxy-2-nitrobenzaldehyde (1 eq.), iodomethane (1.1 eq.) and potassium carbonate (1 eq.) were dissolved in DMF at room temperature and the reaction was stirred for 16 hours. Upon completion of the reaction, the reaction mixture was concentrated and subsequently partitioned between ethyl acetate and water. The organic layer was washed with brine, dried over anhydrous sodium sulfate and concentrated to give 5-methoxy-2-nitrobenzaldehyde in quantitative yield. Mass spectrometry analysis showed the molecular ion peak [M+H]+ = 182.

42454-06-8
215 suppliers
$8.00/1g

74-88-4
357 suppliers
$15.00/10g

20357-24-8
118 suppliers
$8.00/250mg
Yield: 100%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 20; for 16 h;
Steps:
A; 1.1 EXAMPLE 1; Synthesis of (4-{2-[(4-bromophenyl)amino]quinazolin-6-yloxy}-(2-pyridyl))-N-methylcarboxamide; Step 1. Synthesis of 5-methoxy-2-nitrobenzaldehyde 2:
Step 1. Synthesis of 5-methoxy-2-nitrobenzaldehyde 2: A mixture containing 5-hydroxy-2-nitrobenzaldehyde (1 eq) in DMF with iodomethane (1.1eq) and potassium carbonate (1eq) was stirred at room temperature for 16 hours. The resulting mixture was concentrated and partitioned between ethyl acetate and water. The organic layer was washed with brine and dried and concentrated to afford 5-methoxy-2-nitrobenzaldehyde in quantitative yield. MS: MH+=182.
References:
Chiron Corporation US2005/85482, 2005, A1 Location in patent:Page/Page column 13; 17
879-55-0
19 suppliers
$175.00/1 G

20357-24-8
118 suppliers
$8.00/250mg

42454-06-8
215 suppliers
$8.00/1g

77-78-1
319 suppliers
$19.69/1ml

20357-24-8
118 suppliers
$8.00/250mg

42454-06-8
215 suppliers
$8.00/1g
![2-[2-[(E)-2-[4-(dimethylamino)phenyl]ethenyl]-6-methylpyran-4-ylidene]propanedinitrile](/CAS/20180601/GIF/96042-30-7.gif)
96042-30-7
4 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g

20357-24-8
118 suppliers
$8.00/250mg
1882-69-5
289 suppliers
$6.00/5g

20357-24-8
118 suppliers
$8.00/250mg