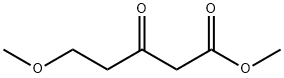
5-METHOXY-3-OXOVALERIC ACID METHYL ESTER synthesis
- Product Name:5-METHOXY-3-OXOVALERIC ACID METHYL ESTER
- CAS Number:62462-05-9
- Molecular formula:C7H12O4
- Molecular Weight:160.17
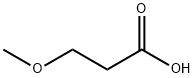
2544-06-1
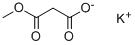
38330-80-2

62462-05-9
1. 3-Methoxypropionic acid (1.81 mL, 19.2 mmol) was suspended in anhydrous acetonitrile (80 mL) and stirred. 2. Methyl 5-methoxy-3-oxopentanoate (3.24 g, 20.0 mmol) was added in one portion and the reaction mixture was stirred at room temperature for 1.5 h. 3. A powdered mixture of magnesium chloride (1.57 g, 16.5 mmol) and potassium methyl malonate (4.5 g, 28.8 mmol) was added in one portion (note that CO2 gas was produced during the reaction). 4. The reaction mixture continued to be stirred at room temperature. 5. MgCl2 (1.57 g, 16.5 mmol) and potassium malonate monohydrate (4.5 g, 28.8 mmol) in a powdered mixture (note that CO2 gas is produced during the reaction). 4. Continue to stir the reaction mixture at room temperature. 5. Vacuum remove the volatiles, then add 2M hydrochloric acid (105 mL). 6. Stir the solution for 1 hour at room temperature, and then extract it with dichloromethane (3 x 100 mL). 7. Combine the organic phases, dry them with MgSO4, filter, and remove the solvents in vacuum. 8. dried, filtered and the solvent was removed in vacuum to afford the target product methyl 5-methoxy-3-oxovalerate (2.69 g, 87% yield).

2544-06-1
104 suppliers
$10.00/1g
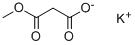
38330-80-2
295 suppliers
$14.00/5g

62462-05-9
76 suppliers
$31.00/100mg
Yield: 87%
Reaction Conditions:
Stage #1:3-Methoxypropionic acid with 1,1'-carbonyldiimidazole in acetonitrile at 20; for 1.5 h;
Stage #2:monomethyl monopotassium malonate with magnesium chloride in acetonitrile at 20;
Steps:
X X. Methyl 5-methoxy-3-oxopentanoate
X. Methyl 5-methoxy-3-oxopentanoate CDl (3.24 g, 20.0 mmol) was added portionwise to a stirred suspension of 3-methoxypropanoic acid (1.81 mi, 19.2 mmol) in anhydrous MeCN (80 mL). The mixture was allowed to stir at rt for 1.5 hrs. A powdered mixture of magnesium chloride (1.57 g, 16.5 mmol) and methyl potassium maionate (4.5 g, 28.8 mmol) was added portionwise (note CO2 gas evolved). The mixture was allowed to stir at rt. Volatiies were removed in vacuo and hydrochloric acid (2M, 105 mL) was added. The solution was stirred at rt for 1 hr and extracted with DCM (3 x 100 mL). The organics were dried over magnesium sulfate, filtered and solvent removed in vacuo to afford methyl 5-methoxy-3-oxopentanoate (2.69 g, 87% yield).
References:
KALVISTA PHARMACEUTICALS LIMITED;SMITH, Alun John;NOVAK, Andrew Richard;EVANS, David Michael;EDWARDS, Hannah Joy;STOCKS, Michael John;DAVIE, Rebecca Louise;MARSH, Sally Louise;HODGSON, Simon Teanby WO2016/83816, 2016, A1 Location in patent:Page/Page column 46
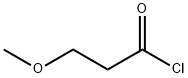
4244-59-1
36 suppliers
$24.00/250mg
126-81-8
424 suppliers
$5.00/10g

62462-05-9
76 suppliers
$31.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

62462-05-9
76 suppliers
$31.00/100mg
110-67-8
220 suppliers
$6.00/25g
96-32-2
346 suppliers
$15.00/5g

62462-05-9
76 suppliers
$31.00/100mg
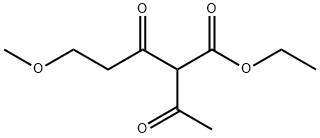
111483-43-3
0 suppliers
inquiry

62462-05-9
76 suppliers
$31.00/100mg