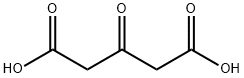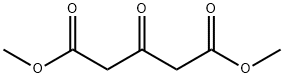
Dimethyl 1,3-acetonedicarboxylate synthesis
- Product Name:Dimethyl 1,3-acetonedicarboxylate
- CAS Number:1830-54-2
- Molecular formula:C7H10O5
- Molecular Weight:174.15

67-56-1
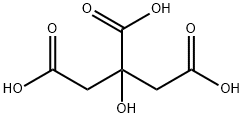
77-92-9

1830-54-2
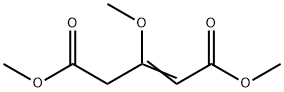
100009-70-9
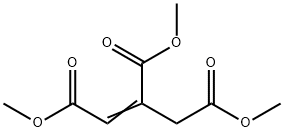
20820-77-3
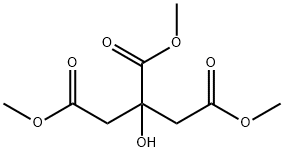
1587-20-8
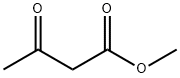
105-45-3
1. 490 kg (370 L) of anhydrous dichloromethane and 1320 kg (753 L) of chlorosulfonic acid were added to a reactor, ensuring that the ratio of dichloromethane to chlorosulfonic acid was 0.5:1 by volume. 2. The temperature of the mixture was adjusted to 10-15 °C and 665.0 kg of citric acid monohydrate was added at a rate of 1.25 kg/min, maintaining the reaction temperature between 10-15 °C. 3. after the addition is complete, continue to stir the reaction mixture at 10-15 °C until no gas is released (at least 6 hours). 4. Cool the reaction mixture to 3-5°C and slowly add 640 kg (800 L) of anhydrous methanol, controlling the internal temperature to no more than 25°C. 5. Warm the reaction mixture to 30-35 °C and stir for 2 hours to complete the esterification reaction. 6. Cool the reaction mixture to 10-12 °C. Slowly add 1300 L of water, controlling the temperature to no more than 15 °C. 7. 800 mL of dichloromethane was added, stirred for 15 minutes and then allowed to stand for 30 minutes to separate the organic phase. 8. The aqueous phase was extracted three times with 400 mL of dichloromethane and the organic phases were combined. 9. The organic phase was washed sequentially with 1000 L of water and 75 kg of sodium bicarbonate in 1000 L of aqueous solution, adjusting the pH to about 7. 10. The organic phase was separated, washed twice with 750 mL of water and evaporated under vacuum. Evaporation conditions: 3-5 kPa, internal temperature not exceeding 75°C. 11. 535 kg of dimethyl 3-oxoglutarate was obtained and used in subsequent reactions without further purification. 12. Gas chromatography analysis conditions: HP-6850 gas chromatograph, HP-1.25 m x 0.32 mm column, film thickness 0.17 μm, carrier gas is hydrogen, programmed temperature increase: 80 ℃ held for 5 minutes, 10 ℃/min to 200 ℃ and held for 8 minutes, detector FID 280 ℃, injector 200 ℃. 13. Expected retention times: 1.18 min for methyl acetoacetate, 5.77 min for dimethyl 3-oxoglutarate, 8.15 min for enol ethers, 10.00 min for trimethyl aconite, 10.42 min for trimethyl citrate, 18.76 min for isosorbic acid derivatives. 14. Area normalization results: methyl acetoacetate 0.31%, dimethyl 3-oxoglutarate 98.33%, enol ether 0.81%, trimethylaconate 0.28%, trimethyl citrate 0.19%, isosorbic acid derivatives not detected, and other contaminants (>0.2%) not detected.

67-56-1
790 suppliers
$9.00/25ml

77-92-9
1726 suppliers
$5.00/10g

1830-54-2
409 suppliers
$9.00/10g

100009-70-9
1 suppliers
inquiry

20820-77-3
16 suppliers
$155.00/500mg

1587-20-8
243 suppliers
$33.00/5g

105-45-3
693 suppliers
$5.00/5g
Yield:1830-54-2 87.3%
Reaction Conditions:
Stage #1: citric acidwith chlorosulfonic acid in dichloromethane at 10 - 15; for 5 - 6 h;
Stage #2: methanol in dichloromethane at 3 - 35; for 2 h;Conversion of starting material;
Steps:
1; 2; 3 Example 1
490 kg (370 1) of anhydrous methylene chloride are added to 1320 kg (753 1) of chlorosulphonic acid (the ratio of methylene chloride to chlorosulphonic acid is 0.5 : 1 by volume). The temperature of the mixture is adjusted to 10-15 oC, and 665.0 kg of citric acid monohydrate are added to it at a rate of 1.25 kg/minute, while the temperature of the reaction mixture is kept between 10 oC and 15 oC. When the addition has been completed the reaction mixture is stirred at a temperature between 10 oC and 15 oC until no more gas is liberated (at least 6 hours). The reaction mixture is then cooled to 3-5 oC and 640 kg (800 1) of anhydrous methanol are added to it at such a rate that the inner temperature does not exceed 25 oC. The reaction mixture is then warmed to 30-35 oC and stirred at the same temperature for 2 hours. The esterifying reaction is completed under this period. The reaction mixture is cooled to 10- 12 oC and 1300 1 of water are added to it at such a rate that the temperature does not exceed 15 oC. To the mixture 800 ml of methylene chloride are added and it is stirred for 15 minutes. The two-phase mixture is clarified for 30 minutes and the organic phase is separated. The aqueous layer is extracted three times with 400 ml each of methylene chloride. The organic phases are combined and washed first with 1000 1 of water, then with a solution of 75 kg of sodium hydrocarbonate in 1000 1 of water. The pH of the solution is adjusted to a value of about 7 by the addition of sodium hydrocarbonate. The organic phase is separated, washed twice with 750 ml each of water and evaporated in vacuo. Evaporation is carried out under a pressure of 3-5 kPa until the inner temperature rises at most to 75 oC. Thus 535 kg of dimethyl 3- OXOGLUTARATE are obtained, which can be used for the further reaction steps without purification. Qualification by gas chromatography: Purity by gas chromatography: Apparatus: gas chromatograph of HP-6850 type Method: Column : HP-1.25 m x 0. 32 MM, 0.17 URN of film thickness Carrier gas: hydrogen Program: 80 oC-5 MINUTES-10 oC/MINUTE-200 oC-8 minutes Detector: FID 280 oC Injector: 200 oC. Expected retention times: Methyl acetoacetate 1.18 minutes Dimethyl 3-oxoglutarate 5.77 minutes Enol ether 8.15 minutes Trimethyl aconitate 10. 00 minutes Trimethyl citrate 10. 42 minutes Isoftalic acid derivative 18.76 minutes Evaluation: by area normalization Result: Methyl acetoacetate: 0.31% Dimethyl 3-oxoglutarate: 98.33% Enol ether: 0. 81% Trimethyl aconitate: 0. 28% Trimethyl citrate : 0. 09% Isoftalic acid derivative :- Other contamination (higher than 0,2 %) Example 2 Into a mixture of 100.0 g (58 ml) of chlorosulphonic acid and 30 ml of methylene chloride 50.0 g of citric acid monohydrate are added within 30 minutes at a temperature between 10 oC and 15 oC. The mixture is stirred at the same temperature for 5 hours, cooled to 3-5 oC and 60 ml of methanol are introduced to it within 15 minutes at such a rate that the temperature does not exceed 25 oC. It is then stirred for 2 hours at a temperature between 30 oC and 35 oC, cooled to 15 oC and 100 ml of water are added to it within 15 minutes. The thus-obtained mixture is extracted three times with 50 ml each of methylene chloride; the organic phases are combined, washed successively with 100 ml of saturated sodium hydrogen carbonate solution and 100 ml of water and evaporated in vacuo. Thus 40.8 g of dimethyl 3- OXOGLUTARATE are obtained. Yield: 98. 5 % Apparatus : gas chromatograph of HP-6850 type Method : Column: HP-1.25 m x 0.32 mm, film thickness of 0. 17 Am Carrier gas: hydrogen Program: 80 oC-5 minutes-10 oC/MINUTE-200 oC-8 minutes Detector: FID 280 oC Injector: 200 oC. Expected retention times: Methyl acetoacetate 1.18 minutes Dimethyl 3-oxo- glutarate 5. 77 minutes Enol ether 8.15 minutes Trimethyl citrate 10.42 minutes Trimethyl aconitate 10.11 minutes Isoftalic acid derivative 18.76 minutes Evaluation: by area normalization Evaluation by gas chromatography (the method was carried out using the parameters specified in Example 1) : Result: Methyl acetoacetate: 0. 49% Dimethyl 3-OXOGLUTARATE : 97. 62% Enol ether: 0.98% Trimethyl citrate: 0. 13% Trimethyl aconitate: 0.29% Isoftalic acid derivative: 0.07% Other contamination (more than 0.2 %) Example 3 (comparative example) Example 1 of the Belgian patent specification No. 879,537 A mixture of 212 ml of chlorosulphonic acid and 600 ml of methylene chloride is prepared (the ratio of methylene chloride to chlorosulphonic acid is 2.83 : 1 by volume). Then 200.0 g of citric acid are added to this mixture within 15-20 minutes, the addition rate of citric acid is 13.3 kg/minute, temperature = 20-22 oC. The reaction mixture is stirred at this temperature for 5 hours, cooled to a temperature between 3"C AND 5 C and 320 ml of methanol are added to it within 15 minutes at such a rate that the temperature remain below 25 oC. The reaction mixture is then stirred for 2 hours at a temperature between 30 oC and 35 oC, cooled to 15 oC, poured into 800 ml of water, stirred vigorously for 15 minutes, clarified for 15 minutes and the phases are separated. The aqueous phase is extracted three times with 150 ml each of methylene chloride. The organic phases are combined, washed once with 400 ml of saturated sodium hydrogen carbonate solution and twice with 200 ml each of water and evaporated in vacuo. Thus 158.2 g of dimethyl 3-oxoglutarate are obtained. Yield: 87. 3 %. Evaluation by gas chromatography (the method was carried out using the parameters specified in Example 1) : Result: Methyl acetoacetate: 0.21% Dimethyl 3-oxoglutarate: 91.07% Enol ether: 4.78% Trimethyl aconitate 0.46% Trimethyl citrate : 2.27% Isoftalic acid derivative: 0.53% Other contamination (higher than 0. 2%)
References:
WO2004/89867,2004,A2 Location in patent:Page 20-26
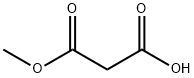
16695-14-0
121 suppliers
$9.00/1g

1830-54-2
409 suppliers
$9.00/10g

77-92-9
1726 suppliers
$5.00/10g

1830-54-2
409 suppliers
$9.00/10g