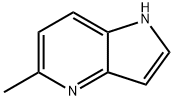
5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE synthesis
- Product Name:5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE
- CAS Number:4943-67-3
- Molecular formula:C8H8N2
- Molecular Weight:132.16
![5-CHLORO-1H-PYRROLO[3,2-B] PYRIDINE](/CAS/GIF/65156-94-7.gif)
65156-94-7
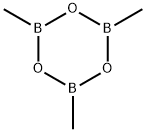
823-96-1
![5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/4943-67-3.gif)
4943-67-3
General procedure for the preparation of 5-methyl-1H-pyrrolo[3,2-b]pyridine: In a microwave reactor, 5-chloro-1H-pyrrolo[3,2-b]pyridine (200 mg, 1.31 mmol), trimethylcyclotriboroxane (0.20 mL, 1.44 mmol) and potassium carbonate (540 mg, 3.93 mmol) were suspended in 5 mL of 1,2-dimethoxyethane. methoxyethane. The mixture was degassed by three vacuum-argon cycles. Subsequently, [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride complex with dichloromethane (53 mg, 0.07 mmol) was added and again degassed by three vacuum-argon cycles. The reaction mixture was stirred at 120°C for 1 h under microwave radiation. After completion of the reaction, it was cooled to room temperature, diluted with ethyl acetate and water, filtered through Celite and eluted with more ethyl acetate and water. The organic phases were combined, washed with brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Purification of the residue using an Isolera purification system (methanol-dichloromethane gradient, 0:100 ascending to 5:95) afforded 5-methyl-1H-pyrrolo[3,2-b]pyridine (95 mg, 0.72 mmol, 55% yield) as an oil with 96% purity. The product was confirmed by 1H NMR (300 MHz, chloroform-d): δ 8.44 (br.s, 1H), 7.60 (d, J = 8.24 Hz, 1H), 7.41 (t, J = 3.02 Hz, 1H), 7.01 (d, J = 8.24 Hz, 1H), 6.69 (br.s, 1H), 2.67 (s, 3H).UPLC/ MS (3 min) analysis showed a retention time of 0.45 min, LRMS: m/z 133 (M + 1).
![5-CHLORO-1H-PYRROLO[3,2-B] PYRIDINE](/CAS/GIF/65156-94-7.gif)
65156-94-7
149 suppliers
$18.00/100mg

823-96-1
187 suppliers
$47.00/5G
![5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/4943-67-3.gif)
4943-67-3
46 suppliers
$60.00/2mg
Yield:4943-67-3 55%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium carbonate in monoethylene glycol diethyl ether at 120; for 1 h;Inert atmosphere;Microwave irradiation;
Steps:
20 PREPARATION 20 5-Methyl-1 H-pyrrolo[3,2-fe]pyridine
PREPARATION 20 5-Methyl-1 H-pyrrolo[3,2-fe]pyridine The title compound of Preparation 19 (200 mg, 1 .31 mmol), trimethylboroxine (0.20 ml, 1 .44 mmol) and potassium carbonate (540 mg, 3.93 mmol) were suspended in 5 ml 1 ,2-dimethoxyethane in a microwave reactor and the mixture was submitted to three vacuum-argon cycles. [1 , 1 '-Bis(diphenylphosphino)ferrocene]dichloropalladium (I I), complex with dichloromethane (53 mg, 0.07 mmol) was added and the resulting mixture was submitted to three further vacuum-argon cycles. The reaction was stirred at 120 °C for 1 h under microwave irradiation. The reaction was allowed to cool, diluted with ethyl acetate and water and filtered through Celite eluting with more ethyl acetate and water. The organic extract was washed with brine, dried over anhydrous magnesium sulphate, filtered and evaporated under reduced pressure. The resulting residue was purified using the Isolera Purification System (methanol-dichloromethane gradient, 0: 100 rising to 5:95) to give 95 mg (0.72 mmol, 55%) of the title compound as an oil. Purity 96%. 1 H N MR (300 MHz, CHLOROFORM-d) δ ppm 8.44 (br. s., 1 H), 7.60 (d, J=8.24 Hz, 1 H), 7.41 (t, J=3.02 Hz, 1 H), 7.01 (d, J=8.24 Hz, 1 H), 6.69 (br. s., 1 H), 2.67 (s, 3 H). UPLC/MS (3 min) retention time 0.45 min. LRMS: m/z 133 (M+1 ).
References:
WO2013/10880,2013,A1 Location in patent:Page/Page column 83-84
400777-06-2
121 suppliers
$45.00/10mg
![5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/4943-67-3.gif)
4943-67-3
46 suppliers
$60.00/2mg
![3-Pyridinamine, 6-chloro-2-[(1E)-2-ethoxyethenyl]-](/CAS/20210111/GIF/1420070-26-3.gif)
1420070-26-3
0 suppliers
inquiry
![5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/4943-67-3.gif)
4943-67-3
46 suppliers
$60.00/2mg
5350-93-6
430 suppliers
$6.00/5g
![5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/4943-67-3.gif)
4943-67-3
46 suppliers
$60.00/2mg
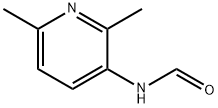
54173-21-6
0 suppliers
inquiry
![5-METHYL-1H-PYRROLO[3,2-B]PYRIDINE](/CAS/GIF/4943-67-3.gif)
4943-67-3
46 suppliers
$60.00/2mg