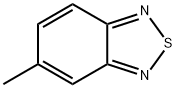
5-METHYL-2,1,3-BENZOTHIADIAZOLE synthesis
- Product Name:5-METHYL-2,1,3-BENZOTHIADIAZOLE
- CAS Number:1457-93-8
- Molecular formula:C7H6N2S
- Molecular Weight:150.2
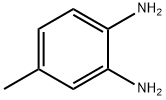
496-72-0

1457-93-8
(1) To a 250 mL three-neck flask was added 4-methylbenzene-1,2-diamine (3.75 g, 30 mmol) and dichloromethane (60 mL) followed by trimethylamine (13.14 g, 130 mmol). The mixture was stirred until 4-methylbenzene-1,2-diamine was completely dissolved. Under stirring, sulfurous dichloride (11.47 g, 100 mmol) was slowly added dropwise and then the reaction mixture was heated to reflux for 5 hours. Upon completion of the reaction, the reaction was quenched with 1 mol/L sodium hydroxide solution and subsequently extracted with dichloromethane (30 mL x 3). The organic phases were combined and concentrated by rotary evaporator. The crude product was purified by silica gel column chromatography using petroleum ether as eluent to afford brown needle-like crystals of 5-methyl-2,1,3-benzothiadiazole (3.69 g, 82% yield). The product characterization data were as follows: melting point 33-34 °C; 1H NMR (CDCl3, 400 MHz, TMS): δ 7.88 (d, 1H, J = 9 Hz), 7.76 (s, 1H), 7.43 (d, 1H, J = 9 Hz), 2.55 (s, 3H); 13C NMR (CDCl3, 100 MHz, TMS): δ 155.31, 153.54, 139.94, 132.41, 120.66, 119.76, 21.91; EIMS [M+]: calculated value 150.0252, measured value 150.0251.
![Benzo[C][1,2,5]thiadiazole-5-boronic acid pinacol ester, 97%](/CAS/GIF/1168135-03-2.gif)
1168135-03-2
46 suppliers
$27.00/250mg

74-88-4
357 suppliers
$15.00/10g

1457-93-8
67 suppliers
$24.00/250mg
Yield:1457-93-8 92%
Reaction Conditions:
with C21H30ClNPPd;potassium tert-butylate in tert-Amyl alcohol at 65; for 18 h;Sealed tube;Inert atmosphere;Glovebox;
References:
Haydl, Alexander M.;Hartwig, John F. [Organic Letters,2019,vol. 21,# 5,p. 1337 - 1341] Location in patent:supporting information

496-72-0
328 suppliers
$9.00/1g

1457-93-8
67 suppliers
$24.00/250mg
106-44-5
502 suppliers
$14.00/5g

1457-93-8
67 suppliers
$24.00/250mg
103-89-9
194 suppliers
$6.00/5g

1457-93-8
67 suppliers
$24.00/250mg
