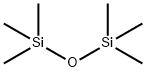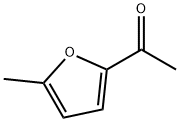
5-Methyl-2-acetylfuran synthesis
- Product Name:5-Methyl-2-acetylfuran
- CAS Number:1193-79-9
- Molecular formula:C7H8O2
- Molecular Weight:124.14
534-22-5

108-24-7

1193-79-9
General procedure for the synthesis of 5-methyl-2-acetylfuran from 2-methylfuran and ethanoic anhydride: continuous acylation of 2-methylfuran (2-MF) by acetic anhydride (AA) on parent Hb, AC-Hb, TA-Hb, Si-Hb zeolites in a fixed-bed reactor (Fig. 1) at atmospheric pressure. The reactor consisted of a glass tube (inner diameter 10 mm) and an electric heater. Before carrying out the acylation reaction, an equal amount of zeolite was activated for 2 h in a drying oven at 200 °C. The activated zeolites need to be transferred to the reactor immediately to avoid exposure to the atmosphere. In standard experiments, the acylation temperature was first controlled at 60 °C by a water bath. Subsequently, 5.9 g of zeolite catalyst was immobilized in the center of the fixed-bed reactor. A mixture of 2-MF and AA (molar ratio of 1:2.5 or 1:4) was delivered to the catalytic column via a metering pump at a set flow rate (0.07 mL/min). Liquid acylation products were periodically collected from the reactor outlet and quantitatively analyzed using a gas chromatograph (GC, Agilent 7890A) equipped with a flame ionization detector (FID) and a DB-FFAP capillary column (30 m length, 0.25 μm film thickness, and 0.25 mm diameter). The target product 2-acetyl-5-methylfuran (2-AC-5-MF) was obtained by acylation reaction and identified using a mass spectrometer (MS, Agilent 5975C) equipped with a mass selective detector.

534-22-5
309 suppliers
$10.00/5mL

108-24-7
0 suppliers
$14.00/250ML

1193-79-9
283 suppliers
$9.00/5g
Yield:1193-79-9 91%
Reaction Conditions:
with HBEA in 1,2-dichloro-ethane at 50; under 760.051 Torr; for 2 h;Reagent/catalyst;Temperature;
Steps:
1.2-3.2 (2) Acetylation of methyl furan to prepare 5-methyl-2-acetyl furan
The methylfuran acetylation reaction is carried out in a fixed bed at atmospheric pressure. 4g of HBEA catalyst was pressed into a tablet, and then sieved to obtain catalyst particles with a particle size of 20-60 mesh. The catalyst is loaded in the reactor. Then weigh 6g of methyl furan and 11.2g of acetic anhydride,Dissolved in 1,2-dichloroethane. After heating the reactor to 50°C,The reaction mixture was introduced into the reactor through a constant flow pump and reacted for 2 hours.After the reaction is over, after the kettle body is cooled to room temperature, the catalyst and the liquid phase product are separated by filtration. The liquid phase products were quantitatively analyzed by gas chromatography-mass spectrometry. Instrument information: Shimadzu QP-2010Ultra; Column information: Rtx-5 Sil MS (30m×0.25mm×0.25μm). Chromatography program setting: from 100°C at 10°C/min to 280°C, hold for 20 minutes. Chromatographic analysis results showed that the conversion rate of methylfuran reached 96percent, and the yield of 5-methyl-2-acetylfuran reached 91percent. The solvent was removed by distillation under reduced pressure to obtain 5-methyl-2-acetylfuran.
References:
East China Normal University;Zhao Chen;Li Bolong;Zhao Lei CN111187238, 2020, A Location in patent:Paragraph 0026; 0031-0033; 0040; 0045-0047; 0054; 0059-0061

67-56-1
790 suppliers
$7.29/5ml-f
![Furan, 2-methyl-5-[1-[(trimethylsilyl)oxy]ethenyl]-](/CAS/20210305/GIF/83796-14-9.gif)
83796-14-9
0 suppliers
inquiry

1193-79-9
283 suppliers
$9.00/5g
1825-61-2
258 suppliers
$15.00/25g
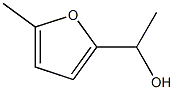
14003-15-7
8 suppliers
inquiry

1193-79-9
283 suppliers
$9.00/5g
98-01-1
509 suppliers
$19.69/250g

1193-79-9
283 suppliers
$9.00/5g