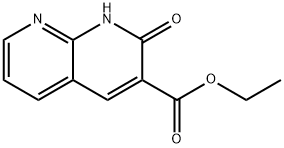
2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:5174-90-3
- Molecular formula:C11H10N2O3
- Molecular Weight:218.21
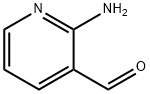
7521-41-7

105-53-3
![2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/5174-90-3.gif)
5174-90-3
General procedure for the synthesis of ethyl 2-oxo-1,2-dihydro-[1,8]naphthyridine-3-carboxylate from 2-amino-3-pyridinecarboxaldehyde and diethyl malonate: 2-amino-3-pyridinecarboxaldehyde (1.0 g, 8.19 mmol) was suspended in a screw-capped pressure tube containing diethyl malonate (6.2 mL, 41.0 mmol). The reaction tube was sealed by the addition of piperidine (1.6 mL, 16.4 mmol). The reaction mixture was heated to 120°C and the reaction was kept at a constant temperature for 3.0 hours. Upon completion of the reaction, the mixture was allowed to cool to room temperature. The precipitate formed was collected by filtration and washed with ether (Et2O) to afford ethyl 2-oxo-1,2-dihydro-[1,8]naphthyridine-3-carboxylate 1.62 g in 91% yield. The structure of the product was confirmed by 1H NMR (CD3OD): δ 8.64 (m, 1H), 8.62 (s, 1H), 8.22 (m, 1H), 7.32 (m, 1H), 4.38 (m, 2H), 1.40 (t, 3H).

7521-41-7
336 suppliers
$6.00/1g

105-53-3
793 suppliers
$5.00/25g
![2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/5174-90-3.gif)
5174-90-3
65 suppliers
$24.00/250mg
Yield:5174-90-3 97%
Reaction Conditions:
with piperidine at 120;
Steps:
Ethyl2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxylate (38).
A solution of commercially available 2-aminonicotinaldehyde(1.00g, 8.19 mmol), diethyl malonate (1.85 ml, 12.18mmol) and few drops of piperidine was stirred at 120°C for 20 hours. After themixture was cooled, the precipitate formed during the reaction was treated withethyl ether and then dried to give a yellow solid (1.74 g, 7.96 mmol, 97%yield). H-NMR (CDCl3): δ 1.42(t, 3H, J=7.2, CH3); 4.44(q, 2H, J= 7.2, CH2);7.27(dd, 1H, J=4.8 Hz, J=8 Hz, Ar); 8.04 (dd, 1H, J=1.6 Hz, J=8 Hz, Ar); 8.47(s, 1H, Ar); 8.90 (dd, 1H, J=1.6 Hz, J=4.8 Hz, Ar); 12.62 (s, 1H, NH) ppm.
References:
Sestito, Simona;Bacci, Andrea;Chiarugi, Sara;Runfola, Massimiliano;Gado, Francesca;Margheritis, Eleonora;Gul, Sheraz;Riveiro, Maria E.;Vazquez, Ramiro;Huguet, Samuel;Manera, Clementina;Rezai, Keyvan;Garau, Gianpiero;Rapposelli, Simona [European Journal of Medicinal Chemistry,2021,vol. 226,art. no. 113895] Location in patent:supporting information
86847-64-5
94 suppliers
$61.00/250mg

105-53-3
793 suppliers
$5.00/25g
![2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/5174-90-3.gif)
5174-90-3
65 suppliers
$24.00/250mg

7521-41-7
336 suppliers
$6.00/1g
2033-24-1
665 suppliers
$6.00/25g

64-17-5
825 suppliers
$10.00/50g
![2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/5174-90-3.gif)
5174-90-3
65 suppliers
$24.00/250mg
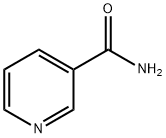
98-92-0
1273 suppliers
$5.00/5g
![2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/5174-90-3.gif)
5174-90-3
65 suppliers
$24.00/250mg
![Pyrido[2,3-d]pyrimidine, 2-(3-pyridinyl)-](/CAS/20180529/GIF/7521-27-9.gif)
7521-27-9
1 suppliers
inquiry
![2-OXO-1,2-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/5174-90-3.gif)
5174-90-3
65 suppliers
$24.00/250mg