
2-Methylamino-1,3-thiazole-4-carboxylic acid ethyl ester synthesis
- Product Name:2-Methylamino-1,3-thiazole-4-carboxylic acid ethyl ester
- CAS Number:57250-86-9
- Molecular formula:C7H10N2O2S
- Molecular Weight:186.23

50-00-0
896 suppliers
$10.00/25g
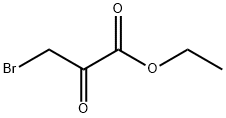
70-23-5
319 suppliers
$6.00/5g
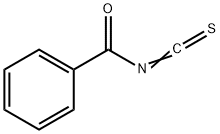
532-55-8
216 suppliers
$9.00/1g

57250-86-9
22 suppliers
$61.00/250mg
Yield:57250-86-9 94%
Reaction Conditions:
Stage #1: formaldehydwith sodium cyanide;ammonium acetate;water at 100; for 0.5 h;Green chemistry;
Stage #2: Benzoyl isothiocyanate at 100; for 0.333333 h;Green chemistry;
Stage #3: ethyl Bromopyruvate at 100; for 1 h;Green chemistry;
Steps:
2.3. General Procedure for the Preparation of Compounds 4
General procedure: The mixture of the aldehydes 1 (2 mmol), ammoniumacetate (5 mmol), NaCN (5 mmol) and KF/CP (10 mol%) inwater (5 mL) at 100 °C stirred for 30 min. After this timebenzoyl isothiocyanate 2 (2 mmol) was added slowly. Thismixture stirred for 20 min. After that, alkyl bromides 3 (2mmol) was added. After 1 h, the reaction completed and itwas confirmed by TLC. Finally, the reaction mixture cooledto room temperature and the solid residue was collected byfiltration. The solid was washed with EtOAc and KF/CP NPsseparated. The solvent was evaporated and the product 4 wasseparated as a solid form and purified by washing with theEt2O.
References:
Hamedani, Naghmeh Faal;Azad, Leila;Shafiee, Shahin;Noushin, Annataj [Combinatorial Chemistry and High Throughput Screening,2021,vol. 24,# 1,p. 88 - 97]
598-52-7
197 suppliers
$6.00/1g
105-36-2
349 suppliers
$5.00/5G

57250-86-9
22 suppliers
$61.00/250mg