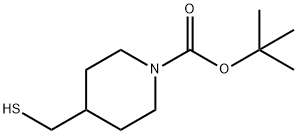
tert-butyl 4-(mercaptomethyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(mercaptomethyl)piperidine-1-carboxylate
- CAS Number:581060-27-7
- Molecular formula:C11H21NO2S
- Molecular Weight:231.35
![1-Piperidinecarboxylic acid, 4-[[(aminoiminomethyl)thio]methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/895126-44-0.gif)
895126-44-0

581060-27-7
A reaction was carried out with tert-butyl 4-((carbamoylimidothio)methyl)piperidine-1-carboxylate (1.2, 15 g, 1.00 eq., crude) as raw material with sodium hydroxide (2.2 g, 55.00 mmol, 1.00 eq.) in a mixture of methanol/water (1:2 v/v, 150 mL) under argon protection at 60 °C with stirring for 2 hours. After completion of the reaction, the reaction mixture was cooled to room temperature. The pH of the reaction solution was adjusted with 35% aqueous hydrochloric acid to 7. The reaction solution was extracted with ethyl acetate (3 x 50 mL) and the organic phases were combined. The organic phase was washed with saturated saline (2 x 50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluent: ethyl acetate/petroleum ether=1:8 v/v) to give 5.6 g of yellow oily product in 44% yield. The structure of the product was confirmed by 1H-NMR (400 MHz, CDCl3): δ 4.13 (m, 2H), 2.69 (m, 2H), 2.46 (m, 2H), 1.82 (m, 2H), 1.50 (s, 9H), 1.32 (m, 1H), 1.18 (m, 2H) ppm.
![1-Piperidinecarboxylic acid, 4-[[(aminoiminomethyl)thio]methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/895126-44-0.gif)
895126-44-0
0 suppliers
inquiry

581060-27-7
46 suppliers
$111.00/50mg
Yield:581060-27-7 5.6 g
Reaction Conditions:
with water;sodium hydroxide in methanol at 60; for 2 h;Inert atmosphere;
Steps:
1 Compound 1.3. tert-Butyl 4-(mercaptomethyl)piperidine-1-carboxylate
A solution of tert-butyl 4-((carbamimidoylthio)methyl)piperidine-1-carboxylate (1.2, 15 g, 1.00 equiv, crude) and sodium hydroxide (2.2 g, 55.00 mmol, 1.00 equiv) in 1:2 (v/v) CH3OH/H2O (150 mL) was stirred for 2 h at 60° C. under argon.
Then the reaction mixture was cooled to room temperature.
The pH value of the solution was adjusted to 7 with HCl(aq) (35%).
The resulting solution was extracted with EtOAc (3*50 mL) and the organic layers were combined.
The organic layer was washed with brine (2*50 mL).
The mixture was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure.
The resulting residue was purified by silica gel chromatography (EtOAc/petroleum ether=1:8 (v/v)) to provide 5.6 g (44%) as yellow oil. 1H-NMR (400 MHz, CDCl3): δ 4.13 (m, 2H), 2.69 (m, 2H), 2.46 (m, 2H), 1.82 (m, 2H), 1.50 (s, 9H), 1.32 (m, 1H), 1.18 (m, 2H) ppm.
References:
US2016/243100,2016,A1 Location in patent:Paragraph 0078
718610-84-5
18 suppliers
inquiry

581060-27-7
46 suppliers
$111.00/50mg
166815-96-9
160 suppliers
$7.00/250mg

581060-27-7
46 suppliers
$111.00/50mg
123855-51-6
407 suppliers
$6.00/5g

581060-27-7
46 suppliers
$111.00/50mg