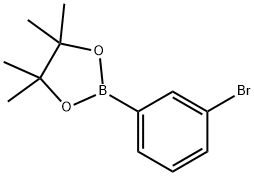
3-Bromo-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-yl)benzene synthesis
- Product Name:3-Bromo-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-yl)benzene
- CAS Number:594823-67-3
- Molecular formula:C12H16BBrO2
- Molecular Weight:282.97
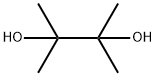
76-09-5
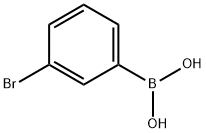
89598-96-9

594823-67-3
The general procedure for the synthesis of 3-bromophenylboronic acid pinacol ester using 3-bromophenylboronic acid and pinacol as raw materials is as follows: 3-bromophenylboronic acid and pinacol were mixed in a suitable solvent under dry reaction conditions, a catalyst (e.g., sulfuric acid or p-toluenesulfonic acid) was added, and the reaction was carried out at room temperature with stirring. After completion of the reaction, the solvent was removed by distillation under reduced pressure, and the crude product obtained was purified by column chromatography to give 3-bromophenylboronic acid pinacol ester in 77% yield, and the product was an oil. Its 1H NMR (300 MHz, CDCl3) data were as follows: δ1.35 (s, 12H), 7.23 (t, 1H), 7.58 (dd, 1H), 7.70 (d, 1H), 7.93 (bs, 1H).
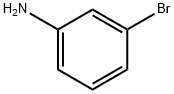
591-19-5
334 suppliers
$10.00/10g

73183-34-3
586 suppliers
$6.00/5g

594823-67-3
101 suppliers
$6.00/250mg
Yield: 80%
Reaction Conditions:
Stage #1:m-Bromoaniline with fluoroboric acid;sodium nitrite in water at 0; for 1 h;Inert atmosphere;Schlenk technique;
Stage #2:bis(pinacol)diborane with methanol at 22 - 25; for 36 h;Inert atmosphere;Schlenk technique;Sealed tube;
Steps:
Synthesis of aryldiazonium tetrafluoroborates
General procedure: An arylamine (50 mmol) was dissolved in 50% hydrofluoroboric acid(17 mL) and water (20 mL). After cooling the reaction mixture to 0 °C, a solution of sodium nitrite (3.4 g in 7.5 mL water) was added dropwise to the reaction system (over 5 min). The resulting mixture was stirred for 1h and the precipitate was collected by filtration. It was redissolved in the minimum amount of acetone and then diethyl ether was added to precipitate the aryl diazonium tetrafluoroborate. The product was filtered, washed with diethyl ether and dried under reduced pressure. Borylation of aryldiazonium salts; general procedure The aryldiazonium salt (0.5 mmol) and (Bpin)2 (0.75 mmol) were added to an oven-dried Schlenk tube. The tube was evacuated and backfilled with argon (three times). CH3OH (0.8 mL) was added to this Schlenk tube. The tube was sealed and the mixture was stirred at room temperature (22-25 °C) for 36 h. After evaporation of the solvent, the residue was purified by column chromatography to afford the product.The arylboronates were purified by chromatography on a silica column eluting with petroleum ether (boiling range 60-90 °C) or a petroleumether/ethyl acetate mixture (ca. 60:1) by volume giving Rf values for the boronates of ca. 0.2-0.3.
References:
Zhang, Xiulian;Zhang, Zhicheng;Xie, Yongbin;Jiang, Yujie;Xu, Ruibo;Luo, Yuhui;Tao, Chuanzhou [Journal of Chemical Research,2018,vol. 42,# 9,p. 481 - 485]

76-09-5
398 suppliers
$8.19/250mg

89598-96-9
345 suppliers
$9.00/1g

594823-67-3
101 suppliers
$6.00/250mg
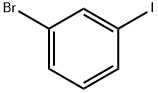
591-18-4
397 suppliers
$5.00/5g

73183-34-3
586 suppliers
$6.00/5g

594823-67-3
101 suppliers
$6.00/250mg

89520-70-7
25 suppliers
$413.00/100mg

73183-34-3
586 suppliers
$6.00/5g

594823-67-3
101 suppliers
$6.00/250mg
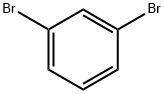
108-36-1
455 suppliers
$10.00/1g

73183-34-3
586 suppliers
$6.00/5g

594823-67-3
101 suppliers
$6.00/250mg