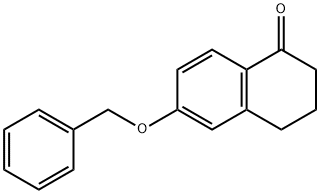
6-(benzyloxy)-3,4-dihydronaphthalen-1(2H)-one synthesis
- Product Name:6-(benzyloxy)-3,4-dihydronaphthalen-1(2H)-one
- CAS Number:32263-70-0
- Molecular formula:C17H16O2
- Molecular Weight:252.31
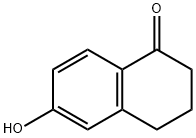
3470-50-6

100-39-0

32263-70-0
General procedure: 6-hydroxy-1-tetralone (0.3 g, 1.85 mmol) was suspended in acetone (15 mL) containing K2CO3 (3.70 mmol). Benzyl bromide (2.035 mmol) was added to the reaction mixture and heated under reflux conditions for 6 hours. The reaction process was monitored by silica gel thin layer chromatography (TLC) using ethyl acetate with petroleum ether (1:2) as mobile phase. Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad and the filtrate was subsequently concentrated under vacuum. The resulting crude product was purified by recrystallization from cyclohexane to give 6-(benzyloxy)-3,4-dihydronaphthalen-1(2H)-one.

3470-50-6
234 suppliers
$6.00/1g

100-39-0
429 suppliers
$10.00/10 g

32263-70-0
121 suppliers
$18.00/100mg
Yield:32263-70-0 97%
Reaction Conditions:
with potassium carbonate in acetone for 6 h;Reflux;
Steps:
3. Synthesis of α-tetralone derivatives 6a-o
General procedure: 6-Hydroxy-1-tetralone (0.3 g, 1.85 mmol) was suspended in acetone (15 mL) containing K2CO3 (3.70 mmol). The reaction was treated with an appropriately substituted arylalkyl bromide (2.035 mmol) and heated under reflux for 6 h. The reaction progress was monitored using silica gel TLC with ethyl acetate:petroleum ether (1:2) as mobile phase. Upon completion, the reaction was filtered through a pad of celite and concentrated in vacuo. The crude thus obtained was purified by recrystallization from cyclohexane.
References:
Legoabe, Lesetja J.;Petzer, Anél;Petzer, Jacobus P. [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 12,p. 2758 - 2763] Location in patent:supporting information

3470-50-6
234 suppliers
$6.00/1g

100-44-7
659 suppliers
$13.50/250G

32263-70-0
121 suppliers
$18.00/100mg
1078-19-9
378 suppliers
$6.00/5g

32263-70-0
121 suppliers
$18.00/100mg