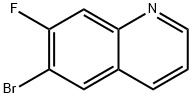
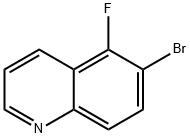
6-Bromo-7-fluoroquinoline synthesis
- Product Name:6-Bromo-7-fluoroquinoline
- CAS Number:127827-52-5
- Molecular formula:C9H5BrFN
- Molecular Weight:226.05
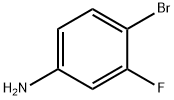
656-65-5

56-81-5

127827-52-5
General procedure for the synthesis of 6-bromo-7-fluoroquinoline from 4-bromo-3-fluoroaniline and glycerol: Intermediate 2: (7-fluoroquinolin-6-yl)methylamine Step 1: Synthesis of 6-bromo-7-fluoroquinoline: 4-bromo-2-fluoroaniline (10 g, 52.62 mmol), ferrous sulfate (3.33 g, 11.97 mmol) and glycerol (15.78 mL) were mixed. Concentrated sulfuric acid (9.15 mL) was added slowly and the reaction mixture was heated to 140 °C. After 12 hours of reaction, the mixture was cooled to 0 °C and the pH was adjusted to 10-12 with 10% sodium hydroxide solution.The reaction mixture was filtered through diatomaceous earth and washed with ethyl acetate to separate the organic and aqueous layers. The organic layer was washed with brine solution, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by column chromatography (eluent: ethyl acetate/petroleum ether) to give the title compound as a white solid (4.9 g, 44% yield). 1H-NMR (δppm, CDCl3, 400MHz): δ 8.96 (dd, J=4.3,2.7Hz, 1H), 8.15 (m, 2H), 7.81 (d, J=9.5Hz, 1H), 7.42 (dd, J=8.3,4.3Hz, 1H).

656-65-5
314 suppliers
$5.00/1g

56-81-5
1771 suppliers
$5.00/25g

127827-52-5
110 suppliers
$18.00/250mg
Yield: 85.8%
Reaction Conditions:
with sulfuric acid;sodium 3-nitrobenzenesulfonate in water at 120 - 140;
Steps:
1 Synthesis step 1: 7-fluoro-6-bromoquinoline
The starting material, 3-fluoro-4-bromoaniline (20 g, 0.105 mol) And catalyst sodium 3-nitrobenzenesulfonate (28 g, 0.124 mol, 1.2 eq) was added to a mixed solution of concentrated sulfuric acid (60 ml) and water (24 ml), heated to an internal temperature of 120 ° C, Glycerol (29 g, 0.315 mol, 3 eq) was added slowly and the reaction was warmed to 130-140 ° C overnight after the addition was complete and allowed to cool. The reaction solution was poured into crushed ice, concentrated ammonia to pH 8, extracted with dichloromethane, washed with water, After drying, column chromatography purified to give a yellow solid 20.3g, yield 85.8%.
References:
Shanghai Pharmaceutical Group Co., Ltd.;Yu Jianxin;Zhao Fei;Hao Yu;Li Ping;Xia Guangxin;Fan Yi CN105968115, 2016, A Location in patent:Paragraph 0560-0562
367-24-8
511 suppliers
$15.00/25 g

56-81-5
1771 suppliers
$5.00/25g

127827-52-5
110 suppliers
$18.00/250mg

656-65-5
314 suppliers
$5.00/1g
98-95-3
34 suppliers
$14.00/25g

127827-52-5
110 suppliers
$18.00/250mg
127827-51-4
24 suppliers
$45.00/2.5mg

656-65-5
314 suppliers
$5.00/1g

56-81-5
1771 suppliers
$5.00/25g

127827-52-5
110 suppliers
$18.00/250mg

127827-51-4
24 suppliers
$45.00/2.5mg