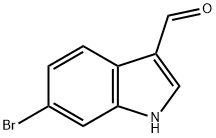
6-Bromoindole-3-carboxaldehyde synthesis
- Product Name:6-Bromoindole-3-carboxaldehyde
- CAS Number:17826-04-9
- Molecular formula:C9H6BrNO
- Molecular Weight:224.05
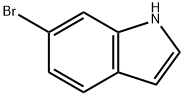
52415-29-9

68-12-2

17826-04-9
6-Bromo-1H-indole-3-carbaldehyde was synthesized as follows: 1) N,N-dimethylformamide (10 mL) was cooled to 0 °C under nitrogen protection and phosphorus trichloride (3.2 mL, 34.6 mmol) was slowly added keeping the reaction temperature between 0 °C and 80 °C. The reaction was carried out by stirring the reaction mixture for 30 min. 2) Stir the reaction mixture at 0 °C for 30 min. 3) Slowly add a solution of N,N-dimethylformamide (28 mL) of 6-bromoindole (5.5 g, 28.1 mmol), keeping the reaction temperature between 0 °C and 10 °C. 4) The ice water bath was removed and the reaction mixture was stirred at room temperature for 2 hours. 5) The viscous reaction mixture was poured into ice water (250 g) and the pH was adjusted to about 7 with 1N sodium hydroxide solution (tested using litmus paper). 6) The mixture was allowed to stand at room temperature overnight. 7) The pink solid was collected by filtration, washed with water, and recrystallized from ethanol to give 6-bromo-1H-indole-3-carbaldehyde (1.6 g, 25% yield) as a light tan solid. 1H NMR (d6-DMSO, 400 MHz): δ 12.20 (br s, 1H), 9.91 (s, 1H), 8.31 (d, J = 3 Hz, 1H), 8.00 (d, J = 9 Hz, 1H), 7.69 (d, J = 2 Hz, 1H), 7.34 (dd, J = 8, 2 Hz, 1H).

52415-29-9
417 suppliers
$9.00/1g

50-00-0
896 suppliers
$10.00/25g

17826-04-9
186 suppliers
$10.00/250mg
Yield:17826-04-9 73%
Reaction Conditions:
with iron(III) chloride;ammonia in water;N,N-dimethyl-formamide at 130; for 5 h;
Steps:
Indolecarbaldehydes 2a-2aa; General Procedure
General procedure: A 50 mL round-bottomed flask equipped with a magnetic stirringbar was charged with the appropriate indole 1 (0.5 mmol,1.0 equiv), 37% aq HCHO (0.5 mmol, 0.0406 g, 1.0 equiv), 25% aqNH3 (1.0 mmol, 0.0681 g, 2.0 equiv), FeCl3 (0.01 mmol, 0.0016 g,2 mol%), and DMF (2 mL). The flask was fitted with a reflux condenser,and the mixture was stirred at 130 °C under open air.When the reaction was complete (TLC), the mixture was cooledto r.t., diluted with sat. aq NaCl (10 mL) and 0.5 M aq HCl (2 mL),and extracted with EtOAc (3 x 7 mL). The organic layers werecombined, washed with sat. aq NaHCO3 (10 mL) and sat. aq NaCl(10 mL), dried (Na2SO4), and concentrated under reduced pressure.The residue was purified by flash column chromatography(silica gel, hexane-EtOAc).
References:
Wang, Qing-Dong;Zhou, Bin;Yang, Jin-Ming;Fang, Dong;Ren, Jiangmeng;Zeng, Bu-Bing [Synlett,2017,vol. 28,# 19,p. 2670 - 2674] Location in patent:supporting information

52415-29-9
417 suppliers
$9.00/1g
100-97-0
0 suppliers
$14.00/250G

17826-04-9
186 suppliers
$10.00/250mg

52415-29-9
417 suppliers
$9.00/1g
100-61-8
400 suppliers
$8.00/1g

17826-04-9
186 suppliers
$10.00/250mg
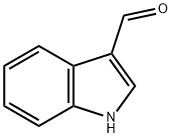
487-89-8
625 suppliers
$6.00/10g
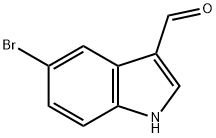
877-03-2
231 suppliers
$6.00/1g

17826-04-9
186 suppliers
$10.00/250mg
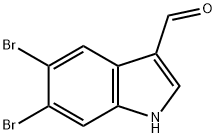
17900-95-7
24 suppliers
inquiry
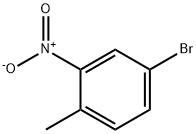
60956-26-5
300 suppliers
$8.00/10g

17826-04-9
186 suppliers
$10.00/250mg