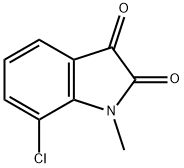
7-chloro-1-methyl-1H-indole-2,3-dione(SALTDATA: FREE) synthesis
- Product Name:7-chloro-1-methyl-1H-indole-2,3-dione(SALTDATA: FREE)
- CAS Number:63220-48-4
- Molecular formula:C9H6ClNO2
- Molecular Weight:195.6
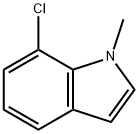
620175-78-2
3 suppliers
inquiry

63220-48-4
15 suppliers
$84.00/250mg
Yield:63220-48-4 93%
Reaction Conditions:
with C7H4IO7S(1-)*K(1+);sodium iodide in water;dimethyl sulfoxide at 60; for 16 h;
Steps:
Oxidation of Indoles 5 to Isatins 6
General procedure: In a 4 mL vial, the starting indole 5 (0.27 mmol) was dissolved in a 2:1 mixture of DMSO and H2O (2.7 mL, 0.1 M). IBX-SO3K (2; 322 mg, 0.81 mmol, 3 equiv) followed by NaI (48 mg, 0.32 mmol, 1.2 equiv) were added to the reaction mixture at r.t. The mixture was then stirred for 16 h at 60 °C. Upon full conversion (as indicated by TLC, eluent: pentanes-EtOAc, 60:40), the mixture was added to H2O (30 mL) and extracted with EtOAc (3 × 10 mL). The combined organic layers were washed with brine (15 mL) and dried (Na2SO4). The solvent was re-moved under reduced pressure and the isatin product 6 was purified by column chromatography over silica gel (Table 1).
References:
Bredenkamp, Angla;Mohr, Fabian;Kirsch, Stefan F. [Synthesis (Germany),2015,vol. 47,# 13,art. no. SS-2015-Z0122-OP,p. 1937 - 1943] Location in patent:supporting information
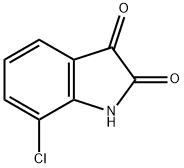
7477-63-6
189 suppliers
$6.00/1g

74-88-4
353 suppliers
$15.00/10g

63220-48-4
15 suppliers
$84.00/250mg

7477-63-6
189 suppliers
$6.00/1g

77-78-1
321 suppliers
$22.00/25g

63220-48-4
15 suppliers
$84.00/250mg
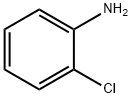
95-51-2
399 suppliers
$5.00/5G

63220-48-4
15 suppliers
$84.00/250mg
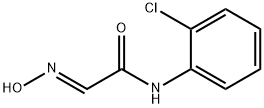
53629-64-4
3 suppliers
inquiry

63220-48-4
15 suppliers
$84.00/250mg