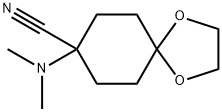
8-DiMethylaMino-1,4-dioxaspiro[4.5]decan-8-carbonitrile synthesis
- Product Name:8-DiMethylaMino-1,4-dioxaspiro[4.5]decan-8-carbonitrile
- CAS Number:65619-92-3
- Molecular formula:C11H18N2O2
- Molecular Weight:210.27
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
489 suppliers
$5.00/1g
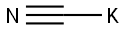
151-50-8
0 suppliers
$43.36/100g

124-40-3
545 suppliers
$18.00/100ml
![8-DiMethylaMino-1,4-dioxaspiro[4.5]decan-8-carbonitrile](/CAS/GIF/65619-92-3.gif)
65619-92-3
15 suppliers
inquiry
Yield:65619-92-3 96%
Reaction Conditions:
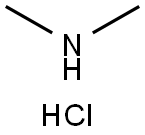
with hydrogenchloride in methanol;water at 0 - 20; for 74 h;Product distribution / selectivity;
Steps:
1; 2 Variant 1: 8-dimethylamino-1,4-dioxa-spiro[4.5]decane-8-carbonitrile
40 percent aqueous dimethylamine solution (116 ml, 0.92 mole), cyclohexane-1,4-dione-monoethylene ketal (30 g, 0.192 mole) and potassium cyanide (30 g, 0.46 mole) were added while cooling with ice to a mixture of 4N hydrochloric acid (50 ml) and methanol (30 ml). The mixture was stirred for 74 hours at room temperature and then extracted with diethyl ether (4×100 ml) after the addition of water (80 ml). After concentration by evaporation the residue was taken up in dichloromethane (200 ml) and dried overnight with magnesium sulfate. The organic phase was concentrated by evaporation and the ketal was obtained in a yield of 97% (40 g) as a white solid with a melting point of 86°-88° C.; Variant 2: 8-dimethylamino-1,4-dioxa-spiro[4.5]decane-8-carbonitrile40 percent aqueous dimethylamine solution (116 ml, 0.92 mole) cyclohexane-1,4-dione-monoethylene ketal (30.0 g, 0.192 mole) and potassium cyanide (30.0 g, 0.46 mole) were added while cooling with ice to a mixture of 4N hydrochloric acid (50 ml) and methanol (30 ml). The mixture was stirred for 74 hours at room temperature and then extracted with diethyl ether (4×100 ml) after the addition of water (80 ml). After concentration by evaporation the residue was taken up in dichloromethane (200 ml) and dried overnight with magnesium sulfate. The organic phase was concentrated by evaporation and the ketal was obtained as a white solid.Yield: 38.9 g (96%)Melting point: 86°-88° C.1H-NMR (DMSO-d6): 1.57 (2H, m); 1.72 (2H; m); 1.85 (2H, m); 1.99 (2H, m); 2.25 (6H, s); 3.87 (4H, m).13C-NMR (DMSO-d6): 30.02; 31.32; 60.66; 63.77; 106.31; 118.40.
References:
US2009/156593,2009,A1 Location in patent:Page/Page column 8
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
489 suppliers
$5.00/1g
506-59-2
556 suppliers
$5.00/5G
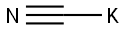
151-50-8
0 suppliers
$43.36/100g

124-40-3
545 suppliers
$18.00/100ml
![8-DiMethylaMino-1,4-dioxaspiro[4.5]decan-8-carbonitrile](/CAS/GIF/65619-92-3.gif)
65619-92-3
15 suppliers
inquiry
![1,4-DIOXA-SPIRO[4.5]DECAN-8-OL](/CAS/GIF/22428-87-1.gif)
22428-87-1
202 suppliers
$6.00/1g

506-59-2
556 suppliers
$5.00/5G
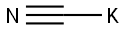
151-50-8
0 suppliers
$43.36/100g
![8-DiMethylaMino-1,4-dioxaspiro[4.5]decan-8-carbonitrile](/CAS/GIF/65619-92-3.gif)
65619-92-3
15 suppliers
inquiry