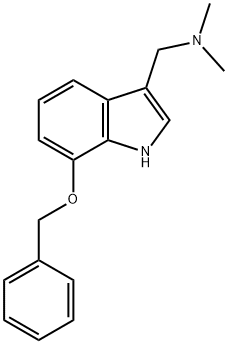
7-BENZYLOXYGRAMINE synthesis
- Product Name:7-BENZYLOXYGRAMINE
- CAS Number:94067-27-3
- Molecular formula:C18H20N2O
- Molecular Weight:280.36
20289-27-4
234 suppliers
$10.00/250mg

50-00-0
896 suppliers
$10.00/25g

124-40-3
546 suppliers
$18.00/100ml

94067-27-3
46 suppliers
$74.50/50mg
Yield:94067-27-3 80%
Reaction Conditions:
with acetic acid in water at 20; for 3.5 h;
Steps:
18 N-((7-Benzyloxy)-1H-indol-3-yl)methyl)-N,N-dimethylamine
To a solution of a 40 % aqueous dimethylamine solution (7.88 g, 69.9 mmol) and a 37 % aqueous formaldehyde solution (5.92 g, 72.9mmol) in acetic acid (70 mL) is added 7-(benzyloxy)-1H-indole (14.2 g, 63.6 mmol) at 0°C under nitrogen atmosphere, and the mixture is stirred at room temperature for 3.5 hours. Water is added to the reaction solution, and the mixture is washed with diethyl ether. The pH value of the aqueous layer is adjusted to pH 12 with a 3N aqueous sodium hydroxide solution, and the mixture is extracted with chloroform. The organic layer is washed with a saturated brine, and dried over anhydrous potassium carbonate. The solvent is evaporated, and the obtained crude product is dissolved in ethyl acetate (100 mL), crystallized from n-hexane (100 mL), and collected by filtration to give N-((7-benzyloxy)-1H-indol-3-yl)methyl)-N,N-dimethylamine (14.3 g, 50.9 mmol, yield: 80 %). 1H-NMR (CDCl3) δ: 8.35 (1H, brs), 7.47-7.49 (2H, m), 7.35-7.43 (3H, m), 7.32 (1H, d, J=8.0Hz), 7.10 (1H, d, J=2.3Hz), 7.03 (1H, dd, J=7.9, 7.7Hz), 6.73 (1H, d, J=7.7Hz), 5.20 (2H, s), 3.61 (2H, s), 2.27 (6H, s).
References:
EP1514869,2005,A1 Location in patent:Page/Page column 31