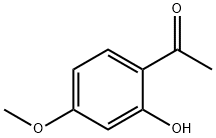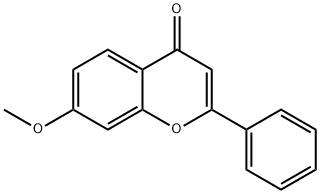
7-Methoxyflavone synthesis
- Product Name:7-Methoxyflavone
- CAS Number:22395-22-8
- Molecular formula:C16H12O3
- Molecular Weight:252.26
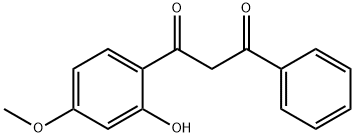
52752-67-7

22395-22-8
1-(2-Hydroxyphenyl)-3-aryl-1,3-propanedione 1a (0.5 g) was mixed with malic acid (1.0 eq.) and placed in an oil bath preheated to 140 °C and reacted for 10 min; or in a microwave reactor for 5 min. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was cooled to room temperature and diluted by adding water (10 mL) and ethyl acetate (10 mL). Subsequently, solid NaHCO3 was added to neutralize the reaction mixture. The organic layer was separated and the aqueous layer was further extracted with ethyl acetate (2 x 10 mL). All organic extracts were combined and dried with anhydrous sodium sulfate and subsequently concentrated under vacuum. The crude product was purified by silica gel column chromatography using a mixed solvent system of hexane-ethyl acetate to give the target product 7-methoxy-2-phenyl-4H-benzopyran-4-one in high yield.
Yield:22395-22-8 88%
Reaction Conditions:
with pyrrolidine;iodine in dimethyl sulfoxide at 150; for 10 h;
Steps:
Typical procedure for the synthesis of flavones:
General procedure: 2'-Hydroxyacetophenone (1 mmol) and substituted aromatic aldehyde (1 mmol) were mixed together along with pyrrolidine (0.5 mmol) and iodine (0.05 mmol) in DMSO solvent (10 mL). The resulting mixture was then heated at 150 °C for the given time. After completion of reaction (monitored by TLC) the reaction mass was allowed to cool and diluted with ethyl acetate (20 mL). Resulting solution was then washed with water and saturated sodium thiosulfate solution followed by drying over anhydrous sodium sulfate and concentrating under reduced pressure to furnish the crude product. The residue obtained was purified by column chromatography using petroleum ether-ethyl acetate as an eluent to afford flavones (3a-r).
References:
Naik, Mayuri M.;Tilve, Santosh G.;Kamat, Vijayendra P. [Tetrahedron Letters,2014,vol. 55,# 22,p. 3340 - 3343]

52752-67-7
4 suppliers
inquiry

22395-22-8
157 suppliers
$11.00/1g
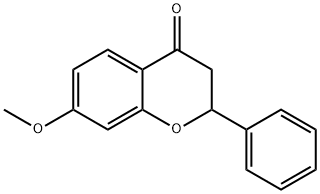
21785-09-1
70 suppliers
$41.00/1g

22395-22-8
157 suppliers
$11.00/1g
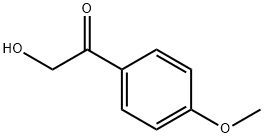
4136-21-4
68 suppliers
$45.00/50mg

100-52-7
972 suppliers
$17.30/2g

22395-22-8
157 suppliers
$11.00/1g
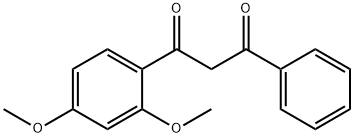
80370-26-9
1 suppliers
inquiry

22395-22-8
157 suppliers
$11.00/1g