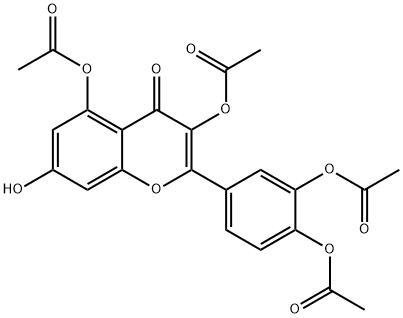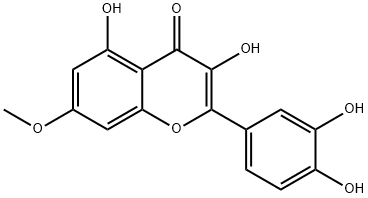
Rhamnetin synthesis
- Product Name:Rhamnetin
- CAS Number:90-19-7
- Molecular formula:C16H12O7
- Molecular Weight:316.26
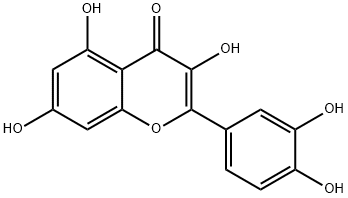
117-39-5
580 suppliers
$5.00/1g

74-88-4
354 suppliers
$15.00/10g
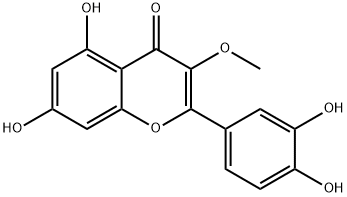
1486-70-0
117 suppliers
$55.00/1 mg
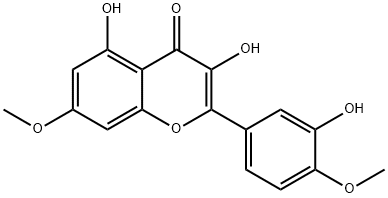
529-40-8
93 suppliers
$120.00/1mg
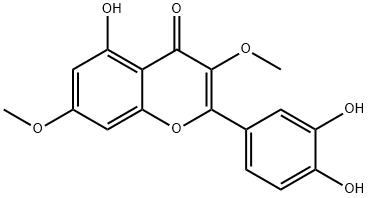
2068-02-2
56 suppliers
inquiry

90-19-7
138 suppliers
$29.00/500μg
Yield:90-19-7 30% ,529-40-8 25% ,2068-02-2 19% ,1486-70-0 19%
Reaction Conditions:
with potassium carbonate in acetone at 20; for 3 h;
Steps:
Preparation of mono- and di-O-methylated analogs ofquercetin (1)
To a mixture of quercetin (1) (200 mg, 0.66 mmol) andanhydrous K2CO3 (114 mg, 0.82 mmol) in acetone (20 mL)was added CH3I (40 μL, d = 2.28 g/mL, 0.66 mmol) dropwise.The mixture was kept stirring at room temperature for2 h, and the progress of the reaction was monitored by TLC.A second lot (20 μL, 0.33 mmol) of CH3I was added andstirring was continued for another 1 h. Water (50 mL) wasadded and the mixture was extracted with EtOAc. Thecombined organic layer was washed with water, dried overanhydrous Na2SO4, and the solvent was evaporated invacuo. The crude products were chromatographed onSephadex LH-20 column eluting with MeOH to give threefractions (A1-A3). Fraction A1 was subjected to columnchromatography over silica gel eluted under isocratic conditionof 100% CH2Cl2 to yield the di-O-methyl ethers 4(34 mg, 25%) and 5 (26 mg, 19%). Fraction A3 wasrepeatedly chromatographed over silica gel eluted underisocratic condition of CH2Cl2-MeOH (10:0.05) to furnishthe mono-O-methyl ethers 2 (39 mg, 30%) and 3 (25 mg,19%), based on the unrecovered starting material, and thestarting material 1 (80 mg).
References:
Pabuprapap, Wachirachai;Wassanatip, Yanisa;Khetkam, Pichit;Chaichompoo, Waraluck;Kunkaewom, Sukanya;Senabud, Pongpan;Hata, Janejira;Chokchaisiri, Ratchanaporn;Svasti, Saovaros;Suksamrarn, Apichart [Medicinal Chemistry Research,2019,vol. 28,# 10,p. 1755 - 1765]

77-78-1
311 suppliers
$19.69/1ml
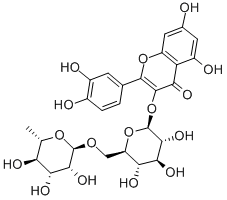
153-18-4
670 suppliers
$6.00/10g
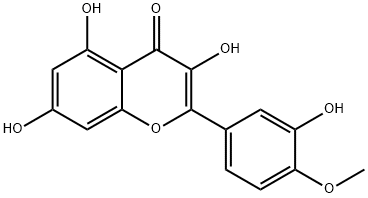
603-61-2
127 suppliers
$45.00/1 mg

529-40-8
93 suppliers
$120.00/1mg

90-19-7
138 suppliers
$29.00/500μg
![2-(2,2-diphenylbenzo[d][1,3]dioxol-5-yl)-3,5,7-trihydroxy-4H-chroMen-4-one](/CAS/GIF/357194-03-7.gif)
357194-03-7
6 suppliers
inquiry

90-19-7
138 suppliers
$29.00/500μg