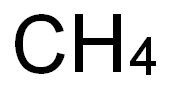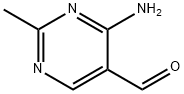
5-Pyrimidinecarboxaldehyde, 4-amino-2-methyl- (6CI,7CI,8CI,9CI) synthesis
- Product Name:5-Pyrimidinecarboxaldehyde, 4-amino-2-methyl- (6CI,7CI,8CI,9CI)
- CAS Number:73-68-7
- Molecular formula:C6H7N3O
- Molecular Weight:137.14
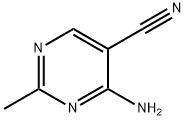
698-29-3
160 suppliers
$7.00/250mg

73-68-7
54 suppliers
inquiry
Yield: 83%
Reaction Conditions:
with formic acid;nickel in water for 0.25 h;Reflux;
Steps:
21 Reference Example 21 4-Amino-2-methylpyrimidine-5-carbaldehyde
(0273) To a solution of the compound of Reference Example 22 (50.0 g, 373 mmol) in formic acid (150 mL) were added water (65 mL) and Raney nickel (50 g). The mixture was heated under reflux for 15 minutes, cooled to room temperature, and filtrated through Celite, and then 28% ammonia water (220 mL) was added thereto under ice cooling. The mixture was stirred under ice cooling for 1 hour, and the precipitate was collected by filtration. The filter cake was washed with water (30 mL) and chloroform (30 mL×2), and dried in vacuo. Furthermore, the filtrate was extracted with chloroform (200 mL) nine times, and the combined organic layer was concentrated. The resulting concentrated residue and the above-obtained filter cake were mixed, chloroform (70 mL) was added thereto, the mixture was stirred at room temperature for 30 minutes, hexane (210 mL) was added dropwise thereto over 10 minutes, and the mixture was stirred at room temperature for additional 1 hour. The precipitate was collected by filtration, washed with hexane/chloroform (3/1, 28 mL), and dried in vacuo to give the title compound (42.6 g, 83%). 1H-NMR (400 MHz, CDCl3) δ: 2.57 (3H, s), 5.98 (1H, brs), 8.15 (1H, brs), 8.57 (1H, s), 9.86 (1H, s).
References:
Sumitomo Dainippon Pharma Co., Ltd.;YOSHINAGA, Hidefumi;URUNO, Yoshiharu;NAGATA, Hidetaka;HASHIMOTO, Masakazu;KATO, Taro US2016/122319, 2016, A1 Location in patent:Paragraph 0272-0273
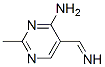
93588-20-6
2 suppliers
inquiry

73-68-7
54 suppliers
inquiry