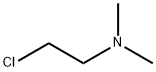
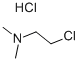4-[2-(Dimethylamino)ethoxy]benzoic acid methyl ester synthesis
- Product Name:4-[2-(Dimethylamino)ethoxy]benzoic acid methyl ester
- CAS Number:73119-82-1
- Molecular formula:C12H17NO3
- Molecular Weight:223.27
Yield:73119-82-1 79%
Reaction Conditions:
with caesium carbonate;potassium iodide in tetrahydrofuran;Heating / reflux;
Steps:
14
This example describes the synthesis of 4- (2-dimethylaminoethoxy) benzoate, 42. To a solution of methyl-4-hydroxybenzoate 32 (3.00 g, 19.7 mmol) in THF (80 mL), dimethylaminoethyl chloride (2.84 g, 19.7 mmol), cesium carbonate (34.78 g, 9. 86 mmol), potassium iodide (65.5 mg, 0.394 mmol) were added and stirred with reflux overnight. After cooling to room temperature, ddH20 was added, extracted with chloroform, dried over magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (10% MeOH/CHC13) to yield 12a (3.47 g, 15.5 mmol) in 79% yield. Rf 0.31 (10% MeOH/CHCl3). 1H NMR (CDCl3) No. : 2.35 (s, 6H); 6. 68 (t, 2H); 3.90 (s, 3H), 6.95 (d, 2H); 8.00 (d, 2H). 13C NMR (CDC13) 5 : 45.90, 51.80, 58.11, 66.18, 114.12, 122.60, 131.51, 162.58, 166.28. LRMS (EI) mass calculated for C12Hl7NO3 : 223.1, found: 223.1.
References:
WO2005/41946,2005,A1 Location in patent:Page/Page column 33
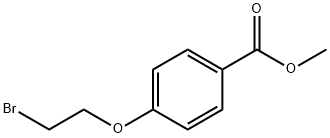
56850-91-0
42 suppliers
$60.00/100mg

124-40-3
545 suppliers
$18.00/100ml
![4-[2-(Dimethylamino)ethoxy]benzoic acid methyl ester](/CAS/20200611/GIF/73119-82-1.gif)
73119-82-1
7 suppliers
inquiry
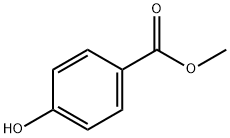
99-76-3
813 suppliers
$5.00/25g
![4-[2-(Dimethylamino)ethoxy]benzoic acid methyl ester](/CAS/20200611/GIF/73119-82-1.gif)
73119-82-1
7 suppliers
inquiry
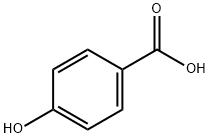
99-96-7
823 suppliers
$5.00/10g
![4-[2-(Dimethylamino)ethoxy]benzoic acid methyl ester](/CAS/20200611/GIF/73119-82-1.gif)
73119-82-1
7 suppliers
inquiry