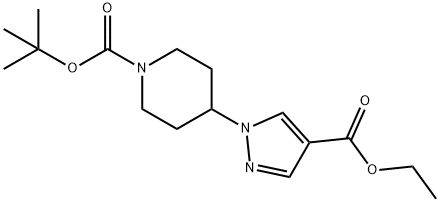
tert-butyl 4-(4-(ethoxycarbonyl)-1H-pyrazol-1-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(4-(ethoxycarbonyl)-1H-pyrazol-1-yl)piperidine-1-carboxylate
- CAS Number:782493-64-5
- Molecular formula:C16H25N3O4
- Molecular Weight:323.39
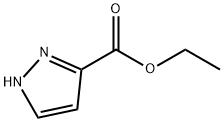
5932-27-4
215 suppliers
$16.00/1g
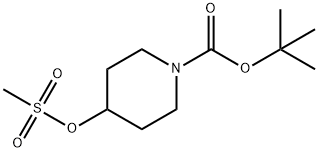
141699-59-4
230 suppliers
$6.00/1g

782493-64-5
18 suppliers
inquiry
Yield: 87%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 5 - 90;
Steps:
2 Step 2: Tert-butyl 4-(4-(ethoxycarbonyl)- I H-pyrazol- l-yl) piperidine- I -carboxyl ate:
To a stirred solution of ethyl-I-H pyrazole carboxylate (5.0 g, 3.7mmol) in DMF (50 mL),Cs2CO3 (23 g, 71.3Smmol) and tert-butyl 4-((methylsulfonyl)-oxy) piperidine-I-carboxylate(9.9 g, 35.6 mmol) were added at 5 °C and reaction mixture was stirred at 90 °C overnight.The completion of the reaction was confirmed by TLC. The reaction mixture was pouredinto ice cold water. The resulting solid was filtered, washed with water (50 mL) and driedunder reduced pressure. It was used in the next step without any further purification. Yield:87% (10 g, off white solid). 1H NMR (400 MHz, DMSO-d6 : 6 8.39 (5, IH), 7.87 (5, IH),4.45-4.39 (m, IH), 4.21 (q, J= 7.2 Hz, 2H), 4.05-4.02 (m, 2H), 2.01-1.98 (m, 2H), 1.85-1.78 (m, 2H), 1.42 (5, 9H), 1.26 (t, J = 7.2 Hz, 3H). LCMS: (Method A) 222.0 (M-Boc), Rt. 2.77 mm, 92.86% (Max).
References:
ASCENEURON S. A.;QUATTROPANI, Anna;KULKARNI, Santosh, S.;GIRI, Awadut, Gajendra WO2017/144633, 2017, A1 Location in patent:Page/Page column 105
37622-90-5
338 suppliers
$6.00/1g

141699-59-4
230 suppliers
$6.00/1g

782493-64-5
18 suppliers
inquiry

37622-90-5
338 suppliers
$6.00/1g
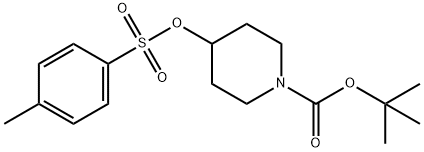
118811-07-7
118 suppliers
$9.00/250mg

782493-64-5
18 suppliers
inquiry

37622-90-5
338 suppliers
$6.00/1g
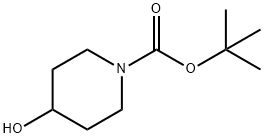
109384-19-2
420 suppliers
$5.00/1g

782493-64-5
18 suppliers
inquiry

109384-19-2
420 suppliers
$5.00/1g

782493-64-5
18 suppliers
inquiry