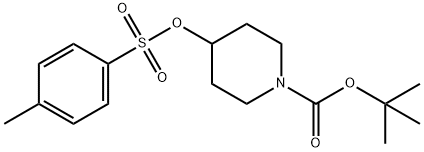
4-(TOLUENE-4-SULFONYLOXY)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-(TOLUENE-4-SULFONYLOXY)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:118811-07-7
- Molecular formula:C17H25NO5S
- Molecular Weight:355.45

98-59-9
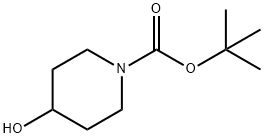
109384-19-2

118811-07-7
To a solution of N-Boc-4-hydroxypiperidine (5 g, 24.8 mmol) in dichloromethane (50 mL) was sequentially added triethylamine (13.9 mL, 99.4 mmol) and p-toluenesulfonyl chloride (9.47 g, 49.7 mmol). The reaction mixture was stirred at room temperature for 20 hours. Upon completion of the reaction, the solution was concentrated in vacuum. The residue was redissolved in a mixture of dichloromethane (20 mL) and water (20 mL) and the organic layer was extracted with dichloromethane (20 mL). Subsequently, the organic layer was washed with 1N HCl (40 mL) and extracted twice more with dichloromethane (2 x 40 mL). The organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated. Finally, the crude product was purified by silica gel column chromatography (petroleum ether/ethyl acetate, 8:2) to afford tert-butyl 4-(toluene-4-sulfonyloxy)piperidine-1-carboxylate (8.7 g, quantitative yield) as a white crystalline solid.

98-59-9
624 suppliers
$9.00/5g

109384-19-2
420 suppliers
$5.00/1g

118811-07-7
118 suppliers
$9.00/250mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 20 h;
Steps:
61.5 Synthesis of compound (34) 1-(tert-butoxycarbonyl)-4-(p-toluensulfonyloxy)- piperidine
To a solution of compound (33) (5 g, 1 eq., 24.8 mmol) in dichloromethane (50 mL) wasadded triethylamine (13.9 mL, 4 eq., 99.4 mmol) and p-toluenesulfonyl chloride (9.47 g, 2eq., 49.7 mmol). The solution was stirred for 20h at room temperature followed by the concentration of this solution in vacuo. The residue was then dissolved in dichloromethane (20 mL) and water (20 mL), extracted with DCM (20 mL), washed with 1 N HCl (40 mL), extracted twice with DCM (2 x 40 mL), dried over Mg504 andevaporated. The resulting mixture was then purified by silica gel column chromatography (Petroleum ether/EtOAc 8:2) and compound (34) was obtained as a white crystalline solid (8.7g, quantitative yield)
References:
CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE;UNIVERSITE D'ORLEANS;AGROFOGLIO, Luigi;ROY, Vincent;PLEBANEK, Elzbieta;BESSIERES, Maxime WO2018/50771, 2018, A1 Location in patent:Page/Page column 72
5382-30-9
38 suppliers
$45.00/10mg

98-59-9
624 suppliers
$9.00/5g

118811-07-7
118 suppliers
$9.00/250mg
86953-81-3
38 suppliers
$514.00/1g

98-59-9
624 suppliers
$9.00/5g

118811-07-7
118 suppliers
$9.00/250mg

98-59-9
624 suppliers
$9.00/5g
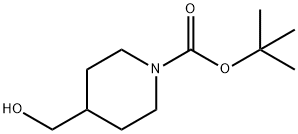
123855-51-6
407 suppliers
$6.00/5g

118811-07-7
118 suppliers
$9.00/250mg

24424-99-5
871 suppliers
$13.50/25G

118811-07-7
118 suppliers
$9.00/250mg