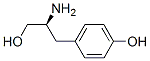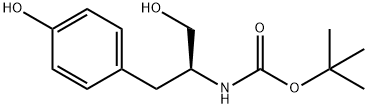
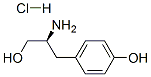
tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate synthesis
- Product Name:tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate
- CAS Number:83345-46-4
- Molecular formula:C14H21NO4
- Molecular Weight:267.32
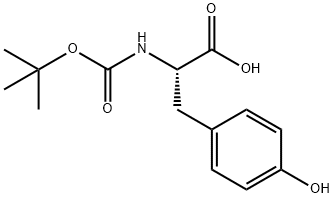
3978-80-1
![tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate](/CAS/20180703/GIF/83345-46-4.gif)
83345-46-4
General procedure for the synthesis of tert-butyl (S)-(1-hydroxy-3-(4-hydroxyphenyl)propan-2-yl)carbamate from N-tert-butyloxycarbonyl-L-tyrosine: 4.1 g of N-tert-butyloxycarbonyl-L-tyrosine (3.6 mmol) was dissolved in 80 mL of anhydrous ether under nitrogen protection. Lithium aluminum hydride (0.4 g, 0.01 mol) was then added in batches. The resulting suspension was heated to reflux and kept overnight. Upon completion of the reaction, it was cooled to room temperature, ethyl acetate was added slowly and the reaction mixture was carefully poured into concentrated sodium hydroxide solution while stirring continuously. The organic layer was separated, washed with water and dried over anhydrous sodium sulfate. After removing the solvent under reduced pressure, 0.4 g of an oily product was obtained.1H NMR (400 MHz, CDCl3) data were as follows: δ1.37 (9H, s), 2.69 (2H, d, J=6.51 Hz), 3.47 (1H, dd, J=5.27,10.85 Hz), 3.57 (1H, dd, J=3.75,10.85 Hz). 3.76 (1H, s), 5.06 (1H, d, J=7.75Hz), 6.70 (2H, d, J=8.37Hz), 6.97 (2H, d, J=8.37Hz), 7.70 (1H, br s) ppm.
4326-36-7
363 suppliers
$6.00/5g
![tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate](/CAS/20180703/GIF/83345-46-4.gif)
83345-46-4
25 suppliers
inquiry
Yield:83345-46-4 99%
Reaction Conditions:
with sodium tetrahydroborate;calcium chloride
References:
McKillop, Alexander;McLaren, Lee;Taylor, Richard J. K.;Watson, Robert J.;Lewis, Norman J. [Journal of the Chemical Society. Perkin transactions I,1996,# 12,p. 1385 - 1393]

24424-99-5
869 suppliers
$13.50/25G
87745-27-5
136 suppliers
$13.00/250mg
![tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate](/CAS/20180703/GIF/83345-46-4.gif)
83345-46-4
25 suppliers
inquiry

3978-80-1
357 suppliers
$7.87/5g
![tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate](/CAS/20180703/GIF/83345-46-4.gif)
83345-46-4
25 suppliers
inquiry
![L-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-, pentachlorophenyl ester (9CI)](/CAS/20210305/GIF/40472-66-0.gif)
40472-66-0
0 suppliers
inquiry
![tert-Butyl [(S)-2-hydroxy-1-(4-hydroxybenzyl)ethyl]carbamate](/CAS/20180703/GIF/83345-46-4.gif)
83345-46-4
25 suppliers
inquiry