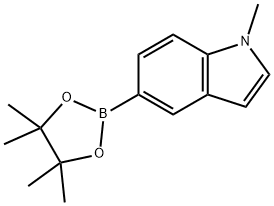
1-Methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole synthesis
- Product Name:1-Methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole
- CAS Number:837392-62-8
- Molecular formula:C15H20BNO2
- Molecular Weight:257.14
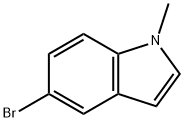
10075-52-2

73183-34-3

837392-62-8
The general procedure for the synthesis of 1-methylindole-5-boronic acid pinacol ester from 5-bromo-1-methyl-1H-indole and biboronic acid pinacol ester is as follows: the synthesis was carried out according to the literature method with appropriate adjustments. To an 8-dram vial equipped with a magnetic stirrer were sequentially added pinacol bis(boronic acid) ester (2 mmol, 508 mg), 5-bromo-1-methyl-1H-indole (2.1 mmol, 441 mg), [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride (0.06 mmol, 44 mg), potassium acetate (6 mmol, 589 mg), and degassed 1,1'-bis(diphenylphosphino)ferrocene(III). ), and degassed 1,4-dioxane (10 mL). The reaction mixture was purged under a strong stream of nitrogen for 5 min to deoxygenate, followed by transferring the reaction system to an oil bath preheated to 80 °C with continuous stirring for 48 h. The reaction was carried out in a cooled room with a temperature range of 0.5 °F to 0.5 °F. Upon completion of the reaction, it was cooled to room temperature and the reaction mixture was filtered through a diatomaceous earth pad and washed with ethyl acetate (50 mL). The filtrates were combined and concentrated under reduced pressure to remove the solvent. The crude product was purified by fast column chromatography with petroleum ether as eluent, followed by a 5% to 12% gradient elution of ether/petroleum ether to afford 342 mg (67% yield) of the target product, 1-methylindole-5-boronic acid pinacol ester, as a white solid.
603-76-9
437 suppliers
$18.00/5g
76-09-5
398 suppliers
$8.19/250mg
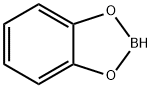
274-07-7
170 suppliers
$78.09/5g

837392-62-8
125 suppliers
$19.00/250mg
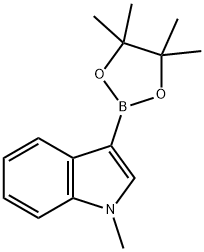
683229-61-0
56 suppliers
$37.00/250mg
Yield: 67 %Spectr. , 13 %Spectr.
Reaction Conditions:
Stage #1:1-methylindole;benzo[1,3,2]dioxaborole with thiophene;tris(pentafluorophenyl)borate in 1,2-dichloro-ethane at 80; for 40 h;Schlenk technique;Inert atmosphere;
Stage #2:2,3-dimethyl-2,3-butane diol with triethylamine in 1,2-dichloro-ethane at 20;Schlenk technique;Inert atmosphere;
Steps:
General Procedure for Catalytic Arene Borylation:
General procedure: In a 20 mL Schlenk tube under Ar, B(C6F5)3 (51.2 mg, 0.10 mmol) was dissolved in distilled1,2-dichloroethane (2 mL), followed by the addition of catecholborane (2, 117 μL, 1.1 mmol) andtetrahydrothiophene (9 μL, 0.10 mmol). Then, to the mixture was added the arene substrate (1.0mmol), and the resulting mixture was heated to 80 °C (oil bath temp.) and stirred for the appropriatereaction time. After cooling to room temperature, excess Et3N (2 mL, ca. 15 mmol) and then, pinacol(355 mg, 3 mmol) were added to the reaction mixture and stirred. The reaction mixture wasquenched by aqueous NH4Cl, extracted with EtOAc, washed with brine, and dried over Na2SO4.Purification by silica gel column chromatography (eluent: CH2Cl2/hexane) was performed to affordthe borylated products.
References:
Kitani, Fumiya;Takita, Ryo;Imahori, Tatsushi;Uchiyama, Masanobu [Heterocycles,2017,vol. 95,# 1,p. 158 - 166]

10075-52-2
119 suppliers
$45.00/1 g

73183-34-3
586 suppliers
$6.00/5g

837392-62-8
125 suppliers
$19.00/250mg
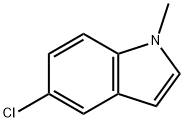
112398-75-1
26 suppliers
$369.00/1g

73183-34-3
586 suppliers
$6.00/5g

837392-62-8
125 suppliers
$19.00/250mg

10075-52-2
119 suppliers
$45.00/1 g
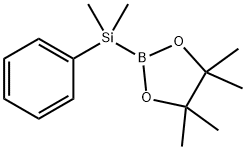
185990-03-8
91 suppliers
$17.00/250mg

837392-62-8
125 suppliers
$19.00/250mg

10075-52-2
119 suppliers
$45.00/1 g
1195-66-0
188 suppliers
$5.00/1g

837392-62-8
125 suppliers
$19.00/250mg