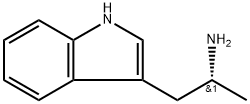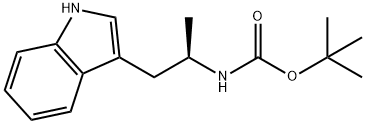
(R)-tert-butyl (1-(1H-indol-3-yl)propan-2-yl)carbamate synthesis
- Product Name:(R)-tert-butyl (1-(1H-indol-3-yl)propan-2-yl)carbamate
- CAS Number:847199-90-0
- Molecular formula:C16H22N2O2
- Molecular Weight:274.36
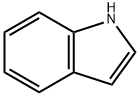
120-72-9
658 suppliers
$6.00/25g
![Tert-Butyl (R)-4-Methyl-2,2-Dioxo-[1,2,3]Oxathiazolidine-3-Carboxylate](/CAS/GIF/454248-53-4.gif)
454248-53-4
135 suppliers
$19.00/250mg

847199-90-0
45 suppliers
inquiry
Yield:847199-90-0 63%
Reaction Conditions:
Stage #1: indolewith methylmagnesium chloride;copper(l) chloride in tetrahydrofuran;dichloromethane at -20 - -15; for 0.333333 h;Inert atmosphere;
Stage #2: (R)-4-methyl-2,2-dioxo-1,2,3-oxathiazolidine-3-carboxylic acid tert butyl ester in tetrahydrofuran;dichloromethane at -10; for 2.5 h;Inert atmosphere;Temperature;Reagent/catalyst;
Steps:
5
[0530] To a mixture of indole (7.4 g, 63.2 mmol, 1.5 equiv.) and CuCl (5.4 g, 54.7 mmol, 1.3 equiv.) in DCM (60 ml) was added MeMgCl (3.0 M in THF, 18.7 mL, 56 mmol, 1.33 equiv.) at - 15°C under N2over 10 min. The resulting light yellow mixture was stirred at -20°C for 10 min, and then a solution of tert-butyl (R)-4-methyl-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide (10.0 g, 42.1 mmol, 1.0 equiv.) in DCM (40 ml) was added dropwise at -10°C under N2 over 30 min. The mixture was stirred at -10°C for 2 hours or until the reaction was complete as judged by TLC (PE/EA = 5/1, disappearance of tert-butyl (R)-4-methyl-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide) or by GC. The reaction was quenched by adding 10% citric acid (100 mL) while maintaining the internal temperature 2SO4(10 g). The resulting mixture was stirred at room temperature for 30 min then filtered. The cake was washed with DCM (50 mL x 2). The filtrate and wash were combined and concentrated under vacuum at 30°C to provide crude tert-butyl (R)-(1-(1H-indol-3-yl)propan-2- yl)carbamate (16.9 g, 40.9 LCA%). Heptane (200 ml) was added to the crude tert-butyl (R)-(1- (1H-indol-3-yl)propan-2-yl)carbamate, and the mixture was stirred at room temperature for 1 hour, during which time, off-white solid precipitated gradually. The solid was collected by filtration, and the cake was washed with heptane (17 mL x 3). The solid was dried under high vacuum at 25 °C to provide 7.0 g of tert-butyl (R)-(1-(1H-indol-3-yl)propan-2-yl)carbamate (25.5 mmol, 97 A%, 99 wt%).1H NMR (400 MHz, DMSO-d6) d 10.76 (s, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.32 (dt, J = 8.1, 1.0 Hz, 1H), 7.09 (d, J = 2.3 Hz, 1H), 7.05 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 6.96 (ddd, J = 8.0, 6.9, 1.1 Hz, 1H), 6.71 (d, J = 8.0 Hz, 1H), 3.76-3.69 (m, 1H), 2.86 (dd, J = 13.9, 5.8 Hz, 1H), 2.64 (dd, J = 14.1, 7.7 Hz, 1H), 1.37 (s, 9H), 1.00 (d, J = 6.6 Hz, 3H).
References:
WO2019/245974,2019,A1 Location in patent:Paragraph 0411; 0526; 0530; 0531; 0532; 0534