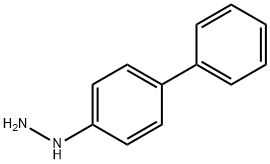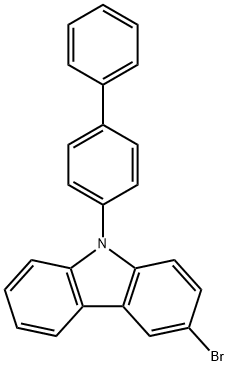
9-[1,1'-Biphenyl-4-yl]-3-bromo-9H-carbazole synthesis
- Product Name:9-[1,1'-Biphenyl-4-yl]-3-bromo-9H-carbazole
- CAS Number:894791-46-9
- Molecular formula:C24H16BrN
- Molecular Weight:398.29
6299-16-7
![9-[1,1'-Biphenyl-4-yl]-3-bromo-9H-carbazole](/CAS2/GIF/894791-46-9.gif)
894791-46-9
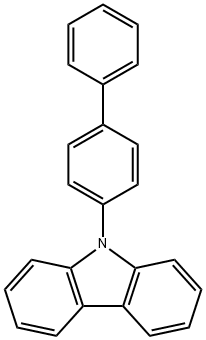
General procedure for the synthesis of 9-[1,1'-biphenyl-4-yl]-3-bromo-9H-carbazole from 9-(4-biphenylyl)carbazole: 3.1 g (10 mmol) of 9-(4-biphenylyl)carbazole was dissolved in 100 mL of chloroform, followed by the slow addition of 1.8 g (10 mmol) of N-bromosuccinimide. The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the organic phase was washed with water. The organic phase was dried by adding anhydrous magnesium sulfate, followed by filtration to remove the desiccant. The filtrate was concentrated to give the crude product, which was dried to obtain 3.7 g (yield: 95%) of the target compound 9-[1,1'-biphenyl-4-yl]-3-bromo-9H-carbazole as a beige powder.
Yield:894791-46-9 95%
Reaction Conditions:
with potassium carbonate;copper(l) chloride in acetonitrile at 0; for 4 h;Schlenk technique;
Steps:
3; 16
Add 0.2mmol (44mg) of biphenyl-4-hydrazine substrate, 0.2mmol (50mg) of 3-bromocarbazole, 1mg (5mol%) of cuprous chloride, 55mg (2eq.) of potassium carbonate to a 50mL Schlenk tube, Acetonitrile 2mL, magnetron was added, and the reaction was stirred at 0°C for 4h. After the reaction is over, add 50 mL of saturated brine, extract three times with 3*50 mL of dichloromethane, add anhydrous sodium sulfate to dry for 30 minutes, and then use a rotary evaporator to remove the low-boiling solvent. The reaction liquid was subjected to column evaporation after rotary evaporation to obtain the target product with a yield of 95%.
References:
CN112010798,2020,A Location in patent:Paragraph 0053-0056; 0099
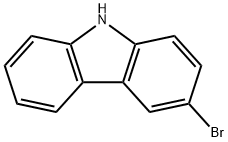
1592-95-6
407 suppliers
$5.00/1g
1591-31-7
339 suppliers
$8.00/5g
![9-[1,1'-Biphenyl-4-yl]-3-bromo-9H-carbazole](/CAS2/GIF/894791-46-9.gif)
894791-46-9
204 suppliers
$5.00/1g
92-66-0
582 suppliers
$10.00/1g
![9-[1,1'-Biphenyl-4-yl]-3-bromo-9H-carbazole](/CAS2/GIF/894791-46-9.gif)
894791-46-9
204 suppliers
$5.00/1g
86-74-8
345 suppliers
$10.00/5g
![9-[1,1'-Biphenyl-4-yl]-3-bromo-9H-carbazole](/CAS2/GIF/894791-46-9.gif)
894791-46-9
204 suppliers
$5.00/1g