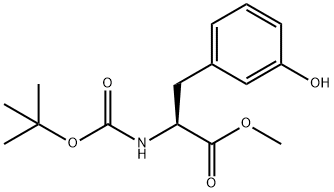
(S)-2-(BOC-AMINO)-3-(3-HYDROXYPHENYL)PROPIONIC ACID METHYL ESTER synthesis
- Product Name:(S)-2-(BOC-AMINO)-3-(3-HYDROXYPHENYL)PROPIONIC ACID METHYL ESTER
- CAS Number:900800-02-4
- Molecular formula:C15H21NO5
- Molecular Weight:295.33

24424-99-5
871 suppliers
$13.50/25G
167935-97-9
13 suppliers
inquiry

900800-02-4
39 suppliers
inquiry
Yield: 0.48 g
Reaction Conditions:
with sodium hydrogencarbonate in methanol at 20; for 8 h;
Steps:
(S)-Methyl-2-(tert-butoxycarbonylamino)-3-(3-hydroxyphenyl)propanoate 11
To a solution of debenzylated ester 10 (1 g, 2.4 mmol) in dichloromethane (3 mL) was added trifluoroacetic acid (1.1 mL, 14.6 mmol) slowly at 0 °C. After 3 h, reaction mixture was concentrated and washed with toluene (to remove the traces of TFA) to give Boc deprotected hydrazine. To the residue (0.5 g, 2.3 mmol) in MeOH (5 mL), activated W-2 Raney-Nickel (30 mg, pre-washed with anhydrous MeOH) and acetic acid (3 drops) were added. The reaction mixture was degassed and saturated with hydrogen (60 psi) for two times. The suspension was stirred at room temperature for 20 h, filtered through celite and the residue was washed thoroughly with MeOH (20 mL) and CH2Cl2 (10 mL). The combined filtrate was concentrated to afford the corresponding amine. To the stirred solution of the crude amine in MeOH (6.0 mL) were added (Boc)2O (0.8 g, 3.5 mmol) and NaHCO3 (until pH becomes basic). Reaction mixture was stirred at room temperature for 8 h. Solvent was evaporated under reduced pressure and the residue was purified by column chromatography (20% ethyl acetate/hexane) to furnish Boc-protected amine 11 as a white solid (0.48 g, 67% over three steps). M.P: 61-62 °C. [α]20D : +37.1 (c = 1.0, CHCl3). IR (KBr): νmax = 3376, 2980, 1744, 1684, 1593, 1515, 1368, 1163, 1059, 772, 698 cm-1. 1H NMR (CDCl3, 500 MHz): δ 7.15 (t, J = 7.7 Hz, 1H), 6.74-6.70 (dd, J = 7.7, 1.9 Hz, 1H), 6.67 (d, J = 7.7 Hz, 1H), 6.64-6.62 (m, 1H), 5.02 (d, J = 7.7 Hz, 1H), 4.61-4.54 (m, 1H), 3.71 (s, 3H), 3.09-3.02 (dd, J = 13.8, 5.6 Hz, 1H), 3.01-2.94 (dd, J = 13.5, 6.7 Hz, 1H), 1.42 (s, 9H) ppm. 13C NMR (CDCl3, 75 MHz): δ 172.5, 156.2, 155.4, 137.4, 129.6, 121.0, 116.1, 114.1, 80.3, 54.3, 52.2, 38.0, 28.2 ppm. HRMS (ESI): calcd. for C15H21NO5Na [M+Na]+ 318.1311; found 318.1307.
References:
Radhika, Laghuvarapu;Chandrasekhar, Srivari [Synthetic Communications,2014,vol. 44,# 24,p. 3602 - 3609] Location in patent:supporting information
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-3-(phenylmethoxy)-, methyl ester](/CAS/20210305/GIF/447461-03-2.gif)
447461-03-2
0 suppliers
inquiry

900800-02-4
39 suppliers
inquiry
626-02-8
304 suppliers
$9.00/5g
93267-04-0
258 suppliers
$5.00/250mg

900800-02-4
39 suppliers
inquiry
1700-37-4
248 suppliers
$9.00/1g

900800-02-4
39 suppliers
inquiry
121056-95-9
3 suppliers
inquiry

900800-02-4
39 suppliers
inquiry