
2-(4-(trifluoromethyl)phenyl)thiazole-5-carbaldehyde synthesis
- Product Name:2-(4-(trifluoromethyl)phenyl)thiazole-5-carbaldehyde
- CAS Number:914348-80-4
- Molecular formula:C10H6FNOS
- Molecular Weight:207.22
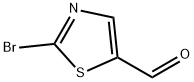
464192-28-7
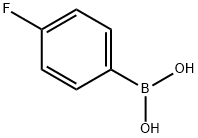
1765-93-1

914348-80-4
General procedure for the synthesis of 2-(4-fluorophenyl)thiazole-5-carboxaldehyde from 2-bromo-5-formylthiazole and 4-fluorophenylboronic acid: 4-fluorophenylboronic acid (7.29 g, 52.08 mmol) was added to a mixture of 2-bromo-5-thiazole-carboxaldehyde (5.0 g, 26.04 mmol) in a solution of toluene (150 mL) and ethanol (75 mL), protected by argon, 2M aqueous sodium carbonate (73.58 mL) and tetrakis(triphenylphosphine)palladium (1.5 g, 1.3 mmol). The reaction mixture was heated and stirred at 85 °C for 6 hours. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure and the residue was diluted with water (150 mL) and extracted with ethyl acetate (5 x 500 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (eluent: hexane solution of 10% ethyl acetate) to give 2-(4-fluorophenyl)thiazole-5-carboxaldehyde as an off-white solid (200 mg, 88% yield).1H NMR (400 MHz, DMSO-d6) δ: 10.06 (s, 1H), 8.74 (s, 1H), 8.14-8.11 (m 2H), 7.39 (t, 2H); LC-MS m/z calculated value [M+H]+ 208.02, measured value 207.9.

464192-28-7
161 suppliers
$12.00/250mg

1765-93-1
550 suppliers
$9.00/1g

914348-80-4
37 suppliers
$60.00/100mg
Yield:914348-80-4 88%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in ethanol;toluene at 85; for 6 h;Inert atmosphere;
Steps:
7.A.1 Step 1: 2-(4-Fluorophenyl)thiazole-5-carbaldehyde
To a solution of 2-bromothiazole-5-carbaldehyde (5.0 g, 26.04 mmol) in toluene (150 mL) and ethanol (75 mL) was added (4-fluorophenyl)boronic acid (7.29 g, 52.08 mmol), 2M sodium carbonate solution (73.58 mL), Pd(PPh3)4 (1.5 g, 1.3 mmol) under argon atmosphere. The resulting mixture was heated at 85°C for 6 h. The reaction mixture was concentrated under reduced pressure, the residue was diluted with water (150 mL), and extracted with ethyl acetate (5 x 500 mL). The organic layer was dried over Na2S04, filtered and concentrated under reduced pressures to obtain crude product. The crude product was purified by Combiflash chromatography (Mobile phase: 10% ethyl acetate in hexane) to give 2-(4- fluorophenyl)thiazole-5-carbaldehyde as off-white solid (200 mg, 88% yield). IH NMR (400 MHz, DMSO-d6): δ 10.06 (s, IH), 8.74 (s, IH), 8.14-8.11 (m, 2H), 7.39 (t, 2H); LC-MS m/z calculated for [M+H]+ 208.02, found 207.9
References:
WO2013/49559,2013,A1 Location in patent:Page/Page column 52

1765-93-1
550 suppliers
$9.00/1g

914348-80-4
37 suppliers
$60.00/100mg