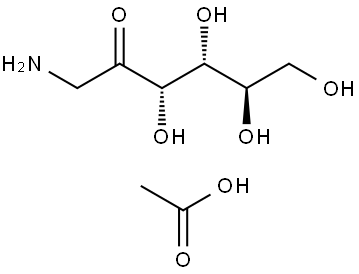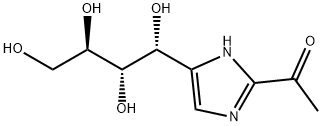
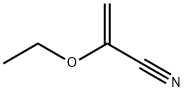
1-[4-(1,2,3,4-TETRAHYDROXYBUTYL)-1H-IMIDAZOL-2-YL]ETHANONE synthesis
- Product Name:1-[4-(1,2,3,4-TETRAHYDROXYBUTYL)-1H-IMIDAZOL-2-YL]ETHANONE
- CAS Number:94944-70-4
- Molecular formula:C9H14N2O5
- Molecular Weight:230.22
Yield: 82%
Reaction Conditions:
Stage #1:2-ethoxyacrylonitrile with sodium methylate in methanol at 20; for 3.25 h;
Stage #2:1-amino-1-deoxy-D-arabino-hexulose acetate in methanol at 20; for 6.25 h;
Stage #3: with methanol;water;sodium methylate;acetic acidmore than 3 stages;
Steps:
2
Example 2 Preparation of 1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-11H-imidazol-2-yl)ethanone To a 3-neck, 12-L round bottom flask equipped with a mechanical stirrer, a temperature controller was added ethoxyacrylonitrile (100.0 g, 1.03 mol) and methanol (HPLC grade, 1.0 L, 10X). To the above stirred solution was added sodium methoxide in methanol (25 wt %, 140.6 ml, 0.60 equiv) in over 15 minutes. The mixture was stirred at 20° C. for at least 3 hours or until 1H NMR showed at least 95% conversion of the ethoxyacrylonitrile to the corresponding imidate). The above solution was transferred to a slurry of fructosamine acetic acid salt (246.9 g, 1.0 equiv, prepared according to Hodge, J. E.; Fisher, B. E., Methods in Carbohydrate Chemistry 11:99-103 (1963)) in methanol (1.0 L, 10X) over 15 min and the mixture was stirred at 20° C. for 6 hours. Another portion of sodium methoxide in methanol (25 wt %, 117.3 ml, 0.50 equiv) was added to the mixture over 10 minutes and the mixture stirred at 20° C. for additional 16 hours. The mixture was then diluted with water (2.0 L, 20X) and treated with acetic acid (118 ml, 2.0 equiv). After stirring at 60° C. for 1 h, the solution was concentrated (50° C., 200 mbar-70 mbar) to 1.2 L total volume. The slurry mixture was cooled to 0° C. and stirred for 1 h, then filtered and the solids were washed with water (100 ml, *2). The solids were collected and dried under vacuum at 50° C. to afford 196.8 g crude product as a pale yellow solid, which was then treated with water (980 ml, 5X) and the resulting slurry was heated to boiling for 15 min, then re-cooled to 0° C. and stirred for an additional 1 h. The mixture was again filtered, and the solids were washed with water (100 ml, *2) and dried to constant weight in a vacuum oven at 50° C. to provide 194.8 g (82%) of the title product (THI) as a pale yellow solid (KF=0.4%). 1H NMR (D2O w/a drop of DCl in D2O) 7.48 (d, J=2.0 Hz, 0.9H), 7.19 (d, J=2.0 Hz, 0.1H), 5.09 (s, 0.9H), 4.98 (s, 0.1H), 3.40-3.70 (m, 4H), 2.53 (d, J=2.4 Hz, 3H); 13C NMR (D2O w/a drop of DCl in D2O) 185.0, 139.4, 138.0, 119.5, 73.0, 70.9, 65.0, 63.2, 26.7; MH+=231.2.
References:
Wu, Wenxue;Yan, Jie;Zhang, Haiming US2008/262241, 2008, A1 Location in patent:Page/Page column 5; 6
19479-65-3
6 suppliers
inquiry
1078691-95-8
13 suppliers
inquiry
![1-[4-(1,2,3,4-TETRAHYDROXYBUTYL)-1H-IMIDAZOL-2-YL]ETHANONE](/CAS/GIF/94944-70-4.gif)
94944-70-4
67 suppliers
$51.00/500μg

19479-65-3
6 suppliers
inquiry
1201794-87-7
1 suppliers
inquiry
![1-[4-(1,2,3,4-TETRAHYDROXYBUTYL)-1H-IMIDAZOL-2-YL]ETHANONE](/CAS/GIF/94944-70-4.gif)
94944-70-4
67 suppliers
$51.00/500μg

1294498-22-8
1 suppliers
inquiry

1078691-95-8
13 suppliers
inquiry
![1-[4-(1,2,3,4-TETRAHYDROXYBUTYL)-1H-IMIDAZOL-2-YL]ETHANONE](/CAS/GIF/94944-70-4.gif)
94944-70-4
67 suppliers
$51.00/500μg

1294498-22-8
1 suppliers
inquiry
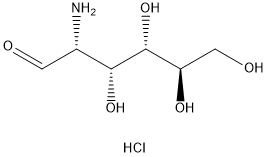
66-84-2
822 suppliers
$6.00/25g
![1-[4-(1,2,3,4-TETRAHYDROXYBUTYL)-1H-IMIDAZOL-2-YL]ETHANONE](/CAS/GIF/94944-70-4.gif)
94944-70-4
67 suppliers
$51.00/500μg