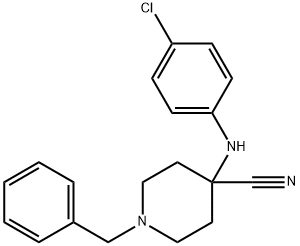
1-benzyl-4-[(4-chlorophenyl)amino]piperidine-4-carbonitrile synthesis
- Product Name:1-benzyl-4-[(4-chlorophenyl)amino]piperidine-4-carbonitrile
- CAS Number:972-20-3
- Molecular formula:C19H20ClN3
- Molecular Weight:325.84
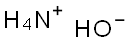
1336-21-6
740 suppliers
$14.56/250ML
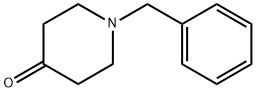
3612-20-2
443 suppliers
$6.00/10g
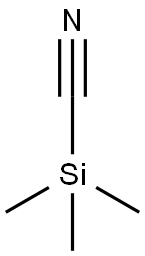
7677-24-9
392 suppliers
$19.00/5g
106-47-8
479 suppliers
$5.00/5G
![1-benzyl-4-[(4-chlorophenyl)amino]piperidine-4-carbonitrile](/CAS/GIF/972-20-3.gif)
972-20-3
7 suppliers
$28.60/250mg
Yield:972-20-3 81%
Reaction Conditions:
in acetic acid;
Steps:
464.1 Step 1
Step 1 1-Benzyl-4-(4-chloro-phenylamino)-piperidine-4-carbonitrile To a solution of 1-Benzyl-piperidin-4-one (2.68 g, 14.20 mmol) in acetic acid (13 ml, glacial) was added 4-Chloro-phenylamine (1.99 g, 15.6 mmol). The reaction was cooled in a cool temperature water bath. Trimethylsilylcyanide (1.89 ml, 14.20 mmol) was added slowly. The reaction mixture was allowed to warm to room temperature and stir overnight under N2. The reaction was cooled to 0° C. Concentrated ammonium hydroxide (15 ml) was added slowly. Next added was cold water. The pH of the mixture was =10. The mixture was extracted three times with methylene chloride, and the organic layers were dried over magnesium sulfate, filtered, and concentrated under reduced pressure. Ether was added to the residue, and the white solid was filtered off and dried under high vacuum to give the titled compound (3.73 g, 81%) 1H-NMR (CDCl3) δ: 1.90-2.01(2H,t), 2.3-2.4(2H,m), 2.45-2.55(2H,t), 2.81-2.93(2H,m), 3.61(2H,s), 3.68(1H,s) 6.87(1H,s), 6.90(1H,s) 7.2-7.38(7H,m). MS m/z: 326 (M+1).
References:
US2005/70549,2005,A1

3612-20-2
443 suppliers
$6.00/10g

7677-24-9
392 suppliers
$19.00/5g

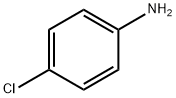
106-47-8
479 suppliers
$5.00/5G
![1-benzyl-4-[(4-chlorophenyl)amino]piperidine-4-carbonitrile](/CAS/GIF/972-20-3.gif)
972-20-3
7 suppliers
$28.60/250mg