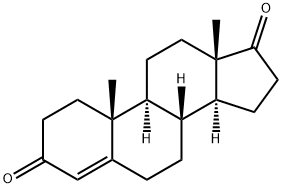
Androstenedione synthesis
- Product Name:Androstenedione
- CAS Number:63-05-8
- Molecular formula:C19H26O2
- Molecular Weight:286.41
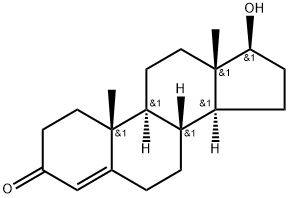
58-22-0
415 suppliers
$21.00/1mg

63-05-8
292 suppliers
$32.00/1mg
Yield:63-05-8 100%
Reaction Conditions:
with Jones reagent in propan-2-one at 0;Jones Oxidation;
Steps:
2
EXAMPLE 2Synthesis of HC1 salt of 10, 13-Dimethyl-l ,6,7,8,9, 10, l l , 12, 13,14, 15, 16-dodecahydro- cyclopenta[a]phenanthrene-3, 17-dione 3-[0-(2-amino-ethyl)-o xime (Hydrochloride salt Compound (12))HC1 salt of Compound (12)Preparation of HC1 salt of Compound (12)To a solution of testosterone (1.7 mmol) in acetone (10 mL) was added an excess of Jones reagent (~2 mL) dropwise, maintaining the temperature at 0 °C. After completion of the reaction (monitored by TLC), iPrOH was added and, after further 10 min, the suspension was filtered through a short plug of silica, washed with acetone and the filtrate evaporated to dryness. The residue was treated with H20 and extracted with EtOAc (3x25 mL). The combined organic extracts were washed with H20, 5% aqueous NaHCC>3 solution, H20, dried over Na2S04 and evaporated to dryness to give the desired product as a white solid. The product was subjected to a short silica column to afford compound al as a white solid in quantitative yield. 'H-NMR (600 MHz, CDC13): δ 5.75 (s, 1H) 2.52 - 1.22 (m, 17H), 1.21 (s, 3H), 1.15 - 0.95 (m, 2H) 0.92 (s, 3H).
References:
WO2011/29639,2011,A2 Location in patent:Page/Page column 104-105
4966-70-5
1 suppliers
inquiry

63-05-8
292 suppliers
$32.00/1mg
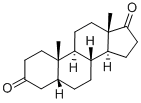
1229-12-5
22 suppliers
inquiry

63-05-8
292 suppliers
$32.00/1mg
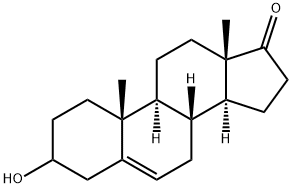
25375-38-6
1 suppliers
inquiry

63-05-8
292 suppliers
$32.00/1mg
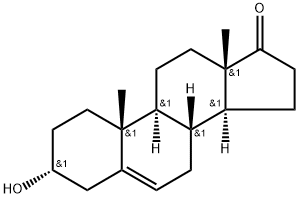
2283-82-1
47 suppliers
inquiry

63-05-8
292 suppliers
$32.00/1mg