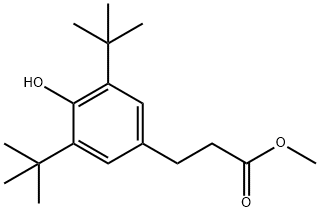
Methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate synthesis
- Product Name:Methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- CAS Number:6386-38-5
- Molecular formula:C18H28O3
- Molecular Weight:292.41
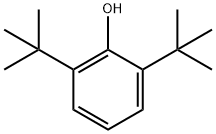
128-39-2
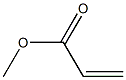
292638-85-8

6386-38-5
(1) 2,6-di-tert-butylphenol (20.6 g), zinc carbonate (mass ratio 1.5) and tributyltin acetate (0.072 g) were added to a three-necked flask fitted with a mechanical stirrer, a nitrogen introduction tube and a dropping funnel under nitrogen atmosphere. The mixture was melted under nitrogen protection and heating conditions to form a reaction system. (2) When the temperature of the reaction system rose to 70°C, methyl acrylate (8.24 g) was slowly added through a dropping funnel. After the dropwise addition was completed, the reaction was continued with stirring for 0.5 hours. The reaction temperature was then raised to 90 °C and maintained for 1 hour. After completion of the reaction, it was cooled to 80 °C and the reaction solution was adjusted to neutral with dilute hydrochloric acid to induce crystallization of the product. After filtration, methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate was obtained in 99% yield and 99.5% purity without recrystallization.

67-56-1
790 suppliers
$7.29/5ml-f
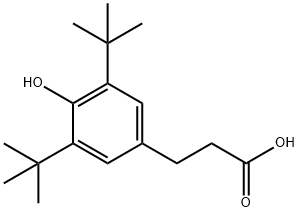
20170-32-5
168 suppliers
$13.00/5g

6386-38-5
180 suppliers
$13.00/10g
Yield:6386-38-5 90%
Reaction Conditions:
with sulfuric acid for 16 h;Reflux;
Steps:
Typical procedure for synthesis of methyl esters
General procedure: According to a literature protocol.[1] Propanoic acid derivative (10 g) was dissolved inmethanol (80 ml) and H2SO4 (700μl, 6 mmol) added. The resulting solution was refluxed for16 h and then allowed to cool to room temperature. The reaction mixture was reduced todryness and re-dissolved in ethyl acetate (20 ml). The organic phase was washed withsaturated aqueous sodium hydrogen carbonate (10 ml), brine (10 ml) and dried over MgSO4.Removal of the solvent gave the desired methyl ester. (Note: column chromatography is oftennot required for the methyl ester starting materials).
References:
Jones, Kevin M.;Hillringhaus, Tim;Klussmann, Martin [Tetrahedron Letters,2013,vol. 54,# 25,p. 3294 - 3297] Location in patent:supporting information
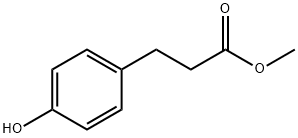
5597-50-2
253 suppliers
$5.00/1g

75-65-0
783 suppliers
$10.00/10ml

6386-38-5
180 suppliers
$13.00/10g
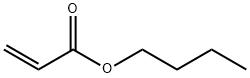
141-32-2
585 suppliers
$18.12/100 mL

128-39-2
363 suppliers
$5.00/25g

292638-85-8
5 suppliers
inquiry
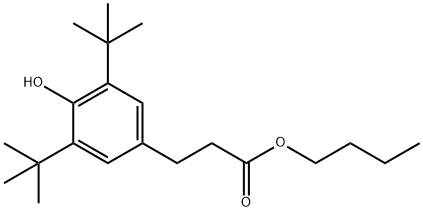
52449-44-2
2 suppliers
inquiry

6386-38-5
180 suppliers
$13.00/10g

67-56-1
790 suppliers
$7.29/5ml-f
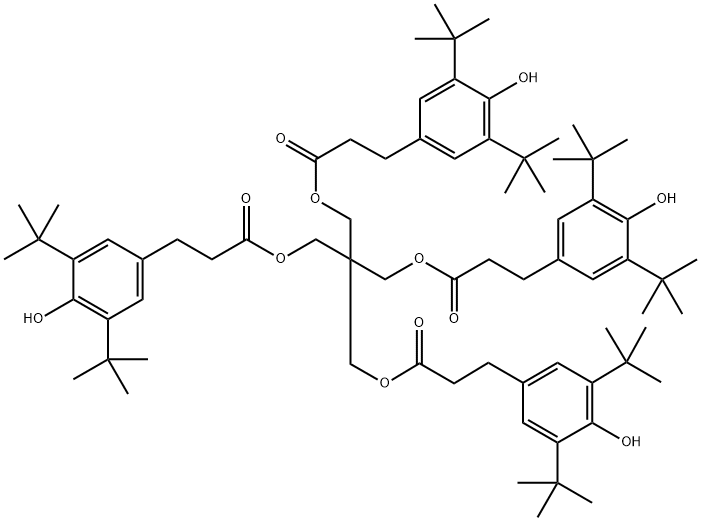
6683-19-8
494 suppliers
$8.00/10g

6386-38-5
180 suppliers
$13.00/10g