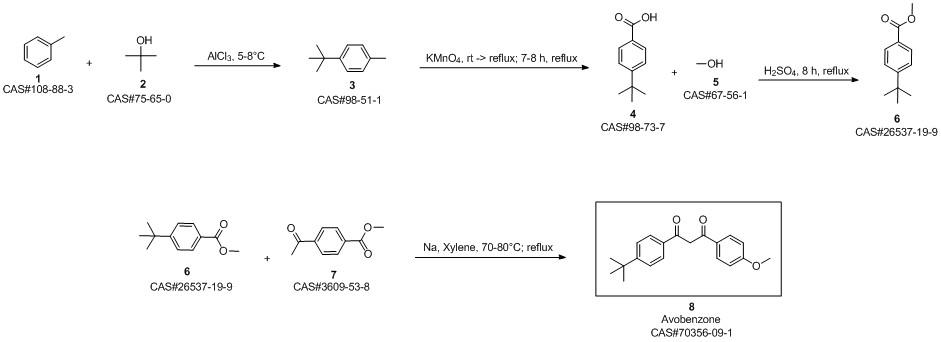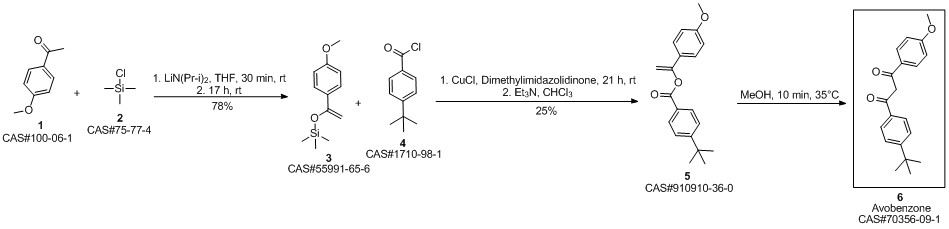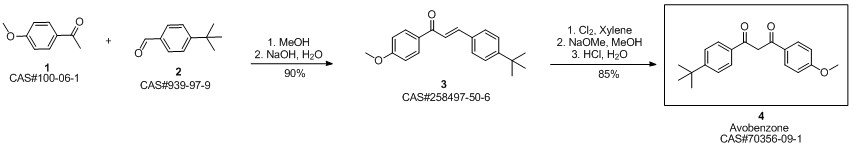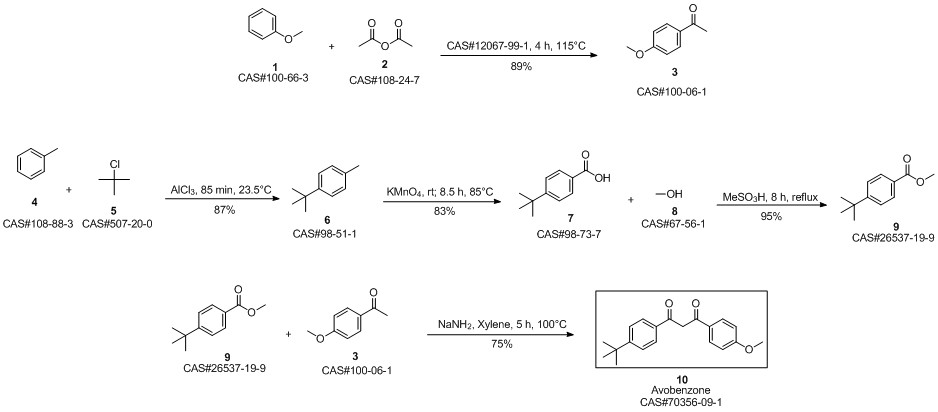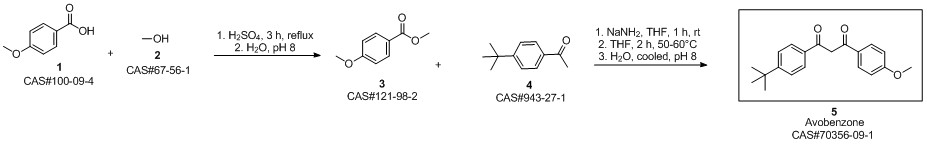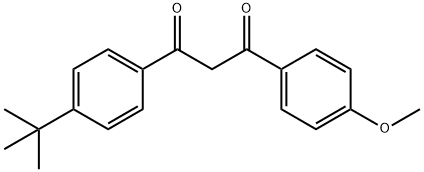
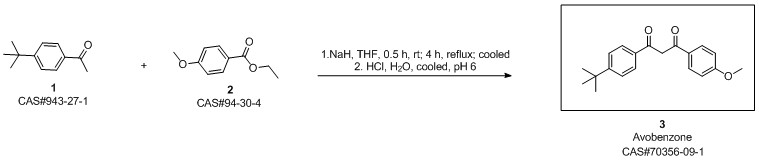
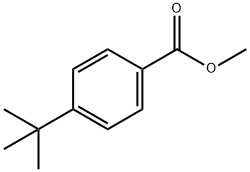
Avobenzone synthesis
- Product Name:Avobenzone
- CAS Number:70356-09-1
- Molecular formula:C20H22O3
- Molecular Weight:310.39
Sun, Dong-Wei; Jiang, Min; Liu, Jin-Tao. Novel Bifunctionalization of Activated Methylene: Base-Promoted Trifluoromethylthiolation of β-Diketones with Trifluoromethanesulfinyl Chloride. Chemistry - A European Journal. Key Laboratory of Organofluorine Chemistry, Shanghai Institute of, Organic Chemistry. University of Chinese Academy of Science, Chinese Academy of Science. Shanghai, Peop. Rep. China 200032. Volume 25. Issue 46. Pages 10797-10802. 2019
26537-19-9
292 suppliers
$16.00/25g
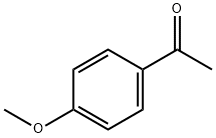
100-06-1
605 suppliers
$18.19/25g

70356-09-1
541 suppliers
$14.00/5g
Yield:70356-09-1 95%
Reaction Conditions:
with potassium methanolate in toluene at 110; under 750.075 Torr; for 2 h;Product distribution / selectivity;
Steps:
1
condensation with various bases In a reaction flask with a 30 ml toluene and the base were provided under a blanket of inert gas. 20 mmole 4-tert-Butyl benzoic acid methyl ester (TBBM) and 20 mmole p-methoxy acetophenone (pMAc) was added. The mixture was stirred at about 110°C/ 1 bar while alcohol and toluene was slowly distilled off (toluene was occasionally replenished to maintain 20 ml volume). After complete conversion of TBBM and pMAc the reaction mixture was cooled and acidified with 2M acetic acid. An aliquot was analyzed by HPLC for the chemical yield of 4-te/f-Butyl-4'-methoxydibenzoylmethane. The results are given in table 1. Table 1 : ResultsAs can be seen from the results in table 1 the use of a potassium alcoholate results in higher yields and shorter reaction times compared to the use of other alkali or earth alkali alcoholates.Furthermore, the potassium salt did not crystallize and remained homogeneously dissolved in toluene whereas the sodium, magnesium and the lithium salt formed a crystalline inhomogeneous mixture
References:
WO2012/84770,2012,A1 Location in patent:Page/Page column 8-9
![2-Propen-1-one, 3-[4-(1,1-dimethylethyl)phenyl]-1-(4-methoxyphenyl)-](/CAS/20210305/GIF/258497-50-6.gif)
258497-50-6
0 suppliers
inquiry

70356-09-1
541 suppliers
$14.00/5g
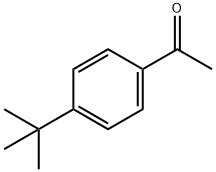
943-27-1
212 suppliers
$6.00/1g
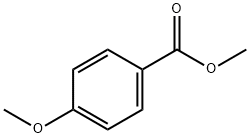
121-98-2
297 suppliers
$12.00/1mg

70356-09-1
541 suppliers
$14.00/5g
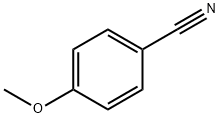
874-90-8
331 suppliers
$8.00/10g
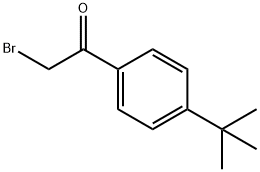
30095-47-7
44 suppliers
$142.00/100mg

70356-09-1
541 suppliers
$14.00/5g